Translate this page into:
Association of sflt-1 as a maternal serum biomarker in preeclampsia: A case–control tertiary care hospital based study
*Corresponding author: Krishnaveni Changalvala, Department of Anatomy, SDUMC, SDUAHER Tamaka, Kolar - 563101, Karnataka, India. krishna.i@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Changalvala K, Kiranmayee P, Raghuveer CV, Sheela SR, Venkateshu KV, Kalyani R. Association of sFlt-1 as a maternal serum biomarker in preeclampsia: A case–control tertiary care hospital-based study. Indian J Med Sci 2021;73:311-6.
Abstract
Objectives:
Preeclampsia (PE) is a multisystemic disorder portrayed by the new beginning of circulatory pressure more noteworthy than 140/90 mmHg and proteinuria with 0.3 g in a 24 h on dip stick emerging after 20 weeks of incubation. The hidden pathophysiology of PE includes endothelial brokenness and vasospasm beginning principally in the placenta. The unusual growth of blood vessels in placenta leads to poor perfusion. This relative hypoxic condition in placenta causes arrival of antiangiogenic factors into the maternal blood dissemination which prompts the modifications in maternal fundamental endothelial functions and causes hypertension. Soluble fms-like tyrosine kinase (sFlt) can form a heterodimer, binding with vascular endothelial growth Factor A and placental growth factor. In preeclamptic subjects, there will be an imbalance in anti-angiogenesis factors and there will be incomplete arterial transformation and cytotrophoblast cell division. Due to imbalance in sFlt levels in preeclamptic women it effects in the blood vessels by constriction and leads to endothelial dysfunction. This study aim is to compare the maternal serum concentration of sFlt levels in normotensive pregnant women to preeclamptic women in early and late gestational weeks.
Material and Methods:
Out of 300 participants in the case–control study, 150 were preeclamptic women as cases and 150 as normotensive pregnant women as controls participated in the present study. A 5 ml of maternal venous blood was collected; the serum was separated and stored at –800°C till the analysis. Using commercially available enzyme-linked immunosorbent assay (ELISA) kits from Chongqing Biospes Co., Ltd., (suppliers: Infobio Company, New Delhi) was measured with ELISA microplate reader at 450 nm (Merilyzer Eiaquant Company).
Results:
Out of 300 participants in the study, 46 pregnant women were early gestational weeks and 254 were late gestational weeks. The complications due to severe PE such as intrauterine death are 15%, intrauterine fetal growth retardation 33%, and premature 15%. The statistical analyses were performed by Statistical Packages for the Social Sciences Software 22. The area under the receiver operating characteristic curve is 0.82, with 91% sensitivity, and 79% specificity. The significance in the maternal serum sFlt levels was calculated by the Mann–Whitney U-test. By comparing the cases and controls, it was found that maternal serum sFlt1 were significantly higher in preeclamptic women with Z = 2.96 and U = 9021 with P = 0.005 significance.
Conclusion:
This is the first South Indian study. If we compare the sFlt1 levels in early and late gestational weeks, in late gestational weeks in controls and PE the levels were highly significant than early gestational weeks of PE and controls. Maternal serum sFlt can be used as a preeclamptic diagnostic marker in South Eastern Kolar population.
Keywords
Angiogenic pregnancy marker
Pregnancy hypertension marker
Maternal circulating Flt-1
Vascular endothelial growth factor receptor-1
Placental diagnostic marker
INTRODUCTION
The human placenta is a multifunctional organ that grows and gets adapted to the increased fetal demand. Throughout pregnancy, the placenta is exposed to physiological and pathological stress. However, these pathological variations are not so evident at the early stages of pregnancy and further lead to complications such as preeclampsia (PE) in mother and fetus results in fetal death, and growth retardation. So to get rid of this placental stress, the only way is to end the pregnancy.[1] PE is a pregnancy-specific syndrome involving different systems associated with endothelial dysfunction and vascular inflammation.[2] Studies have suggested that changes in circulating angiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt1) play a key role in the pathogenesis of PE.[2] The antiangiogenic marker especially sFlt1 contribute to placental stress and effects on the maternal endothelium.[1]
sFlt1 is a glycosylate anti-angiogenic protein. It is derived from the trophoblast of the Flt 1 gene.[3] Due to lack of transmembrane sFlt1 moves freely in maternal circulation and the presence of an extracellular domain binds with vascular endothelial growth factor (VEGF) and placental inhibitor growth factor (PIGF).[4,5]
In the early stages of a normal pregnancy, the uterine endometrial and myometrial spiral arteries invade along with cytotrophoblast for effective placental vascular remodeling. This process is initiated at 12 weeks of gestation and by 18– 20 weeks this remodeling will be completed. This vascular remodeling ensures proper blood supply to the fetus.[3] In humans, the physiological function of sFlt1 is to maintain placental vasculature and to regulate the formation of new blood vessels (at the embryonic stage) especially in tissues such as the kidney, cornea, and uterus.[4,5]
In PE, cytotrophoblast cells fail to transform the arteries which leads to the failure of placental vascular remodeling affects the blood supply/nutrition to the fetus.[3] At 20 weeks of gestation, the angiogenesis is completed. This might be a reason for PE which is evident after 20 weeks of gestation only.[6] Evidence suggests that placental ischemia in PE results in defective placental vascular remodeling.[5] Due to this, the function of sFlt1 was impaired and directly effects on maternal endothelium and counterpart in the initiation of PE. Thus, sFlt1 plays a key role in the pathophysiology of PE.[1,7] It is evident that there was a great variation in elevated levels of sFlt1 apart from genetic factors, environmental susceptibility, racial difference, geographical, and socioeconomic factors were added to that.[3] The prediction of PE especially in high-risk pregnancies remains a question to clinicians, due to paucity in data regarding limited specific marker or single test that is specific in prediction and effective enough for PE detection.[6] In this regard, the study aims to compare the maternal serum concentration of sFlt1 levels in normotensive pregnant women to preeclamptic women in early and late gestational weeks.
MATERIAL AND METHODS
The study protocol was approved by the central ethical committee. The samples were collected over a period from August 2017 to October 2018 after taking written informed consent from each participant in this study. Both the cases (PE subjects) and normotensive subjects were from the Department of Obstetrics and Gynaecology at R. L. Jalappa Research Institute and Teaching Hospital.
Study design and study participants
A total of 348 pregnant women participated in the present study. About 16% of pregnant women were dropped out of the study. The reasons for drop out are 10% of subjects were shifted to their home town for delivery during 33–35 weeks (parents’ house). About 4% of subjects were not interested to give the blood sample but shared the history so dropped out and the remaining 2% later developed gestational diabetes before delivery (30–33 weeks). Hence, a total of 300 pregnant women with ≥ 20th week of gestation have participated in the present study.
Diagnostic criteria of PE
On the American College of Obstetrics and Gynaecologists were diagnosed as preeclamptic women with ≥ the 20th week of gestation. PE was defined as advance-onset of hypertension after 20 weeks of gestation with blood pressure ≥140/90 mmHg and proteinuria in urine on dipstick of ≥1+. In the absence of proteinuria, PE can be considered as severe hypertension (Blood Pressure ≥160/110) and laboratory abnormalities consistent with hemolysis, low platelets, and elevated liver enzymes (HELLP syndrome) or symptoms (i.e., headache, visual changes, and right upper quadrant pain). Creatinine ≥ 1.1 mg/dL is diagnosed as renal insufficiency. Early gestation is defined as delivery before 34 weeks and late gestation is delivery after or at 34 weeks (34–37 weeks).[8]
Exclusion criteria
The Gravida women with (gestational) hypertension, chronic hypertension, (gestational) diabetes, previous history of more than 2 abortions, previous pregnancy with an anomalous fetus, and thrombophilia like disorders were excluded from the study.
Inclusion criteria
Gravida women with 20th week of gestation who were diagnosed as PE, both primigravida and multigravida, and who were healthy normotensive pregnant women after the 20th week of gestation without any complications till delivery were controls.
Sample collection and analysis
The 5 ml of maternal venous blood under complete aseptic precautions blood was collected in red-capped vacutainers. The serum was separated by centrifugation and stored at –80°C till analysis gradually brought to –20°C and then to 4°C and then to room temperature. As it is not done routinely in our laboratory so, by purchasing (self-financing) commercially available enzyme-linked immunosorbent assay (ELISA) kits from Chongqing Biospes Co., Ltd., (suppliers: Infobio Company, New Delhi), the serum samples were measured by following strictly as per the protocol. The maternal serum analysis was further estimated with an ELISA microplate reader at 450 nm (Merilyzer Eiaquant Company).
Statistical analysis
The results were analyzed by Statistical Package for the Social Sciences Software (SPSS) after verifying normal distribution. Due to non-distribution in data, the statistical analysis was performed by non-parametric test. Hence, results were analyzed by the Mann–Whitney unpaired test. If data are normally distributed, then the cutoff value is calculated based on the mean. Due to non-distribution in data, the cutoff value was calculated by the median in SPSS (version 22.0; SPSS Inc, Chicago, IL, USA). The sensitivity and specificity were interpreted as receiver-operating characteristics of area under curves using SPSS (version 22.0; SPSS Inc, Chicago, IL, USA).
RESULTS
The statistical analysis in samples (cases and controls) was analyzed for 300 subjects. Further, based on gestational weeks, the pregnant women with early gestational weeks were 46 and late gestation was 254 participants. According to their gravida status primigravida was 149 and multigravida was 151 subjects, the details are given in Table 1. The severe PE was 68% and the remaining were mild PE which is depicted in Table 1. After analyzing the normal distribution curve, the statistical significance of the maternal sFlt1 levels in cases and controls was calculated by the Mann–Whitney U-test explained in Table 2. The maternal age, gestational weeks, systolic, and diastolic blood pressures in cases, and controls were analyzed by the Mann–Whitney U-test explained in Table 3. By comparing cases, and controls the maternal serum sFlt1 levels were significantly higher in PE cases (Z = 2.96) U = 9021. The P-value is significant with (≤0.05) < 0.00298 especially if we compare with early and late gestational weeks in late gestational weeks the sFlt1 levels were highly significant. The area under the receiver-operating characteristics curve value is 0.82, 91% sensitivity, and 79% specificity is observed in Table 2 and Figure 1. Due to non-distribution in data, the cutoff value was calculated based on the median by SPSS (version 22.0; SPSS Inc, Chicago, IL, USA). The cutoff value in controls (median) is 320 pg/ ml (Interquartile range 67.62–10). In PE the median cutoff value is 1535.1 ± 237.5 pg/ml (Interquartile range 958.1– 160 = 798.1). In 52.6% of primigravida were preeclamptic patients so this reveals PE is more prone to primigravidas. If compared to the median values of maternal age in cases and controls there was no much difference between early and late gestation, but in gestational age, we found a significant difference in controls and cases when compared to late and early PE. In late PE, the gestational weeks are 37 and whereas in subjects with early PE was 30 weeks only and in controls, late gestation was extended to 39 weeks. Hence, this indicates that due to PE there were more chances of early delivery which affects the growth of the fetus also. If we compare the serum sFlt1 levels in early and late PE, in early gestational weeks of PE the serum sFlt1 levels are high in maternal circulation compared to late gestational weeks. In late gestational weeks in controls and PE, the levels were highly significant than early gestational weeks of PE depicted in Table 4.
| Gravida status | Number of PE subjects (n=150) (%) | Number of normotensive subjects (n=150) (%) |
|---|---|---|
| Primigravida | 79 (52.6) | 70 (46.6) |
| Multigravida | 71 (47.3) | 80 (53.3) |
| Early Gestation | 36 (24) | 10 (6.6) |
| Late Gestation | 114 (76) | 140 (93.3) |
n: Number of subjects, Cases-Preeclamptic pregnant women and Controls – Normotensive pregnant women, PE: Preeclampsia.
| Preeclampsia and clinical outcomes | Number of cases (n=150) (%) |
|---|---|
| Mild preeclampsia | 48 (32) |
| Severe preeclampsia | 102 (68) |
| Severe preeclampsia + Intrauterine growth retardation/Restriction | 50 (33) |
| Severe preeclampsia + Premature birth | 23 (15) |
| Severe preeclampsia + Intra uterine death | 23 (15) |
| Content | Median±SE | IQ Range | AUC value | Sensitivity | Specificity | P-value |
|---|---|---|---|---|---|---|
| In both cases and controls | 42.5±12.8 | 81−13=68 | 0.82 | 91% | 79% | U = 9021 |
| Z = 2.96 | ||||||
| <0.00298 | ||||||
| PE | 1535.1±237.5 | 958.1−160=798.1 | NA | |||
| Controls | 320±80.2 | 67.62−10=57.62 | ||||
| Severe PE | 1490±350.4 | 340−109.25=230.75 | NA | |||
| Mild PE | 157.5±48.8 | 353.7−105.8=247.8 |
| Content | Gestational weeks | Median±SE | Pvalue |
|---|---|---|---|
| Maternal age | Early gestational weeks in PE cases | 25±0.616 | 0.1336 |
| Early gestational weeks in controls | 23±1.13 | ||
| Late gestational weeks in PE cases | 24±0.40 | 0.1141 | |
| Late gestational weeks in controls | 24±0.267 | ||
| Soluble FLT-1 levels | Early gestational weeks in PE cases | 1595.0±549.5 | 0.00022 |
| Early gestational weeks in controls | 200±170 | ||
| Late gestational weeks in PE cases | 1501±3360 | 0.00001 | |
| Late gestational weeks in controls | 320±80.8 | ||
| Systolic blood pressure | Early gestational weeks in PE cases | 160±4.18 | 0.00001 |
| Early gestational weeks in controls | 115±4.216 | ||
| Late gestational weeks in PE cases | 110±3.39 | 0.00001 | |
| Late gestational weeks in controls | 120±0.757 | ||
| Diastolic blood pressure | Early gestational weeks in PE cases | 110±3.39 | 0.00001 |
| Early gestational weeks in controls | 70±2.62 | ||
| Late gestational weeks in PE cases | 100±1.44 | 0.00001 | |
| Late gestational weeks in controls | 70±0.586 | ||
| Gestational age | Early gestational weeks in PE cases | 30±0.384 | 0.8807 |
| Early gestational weeks in controls | 29.5±0.87 | ||
| Late gestational weeks in PE cases | 37±0.180 | 0.00001 | |
| Late gestational weeks in controls | 39±0.128 |
Significant with P ≤ 0.0001, n: Number of subjects, SD: Standard deviation, SE: Standard error, PE: Preeclampsia. Numbers of subjects involved in the study were as follows: Early gestational weeks in PE cases n=36, early gestational weeks in controls n=10, late gestational weeks in PE cases n=114, late gestational weeks in controls n=140
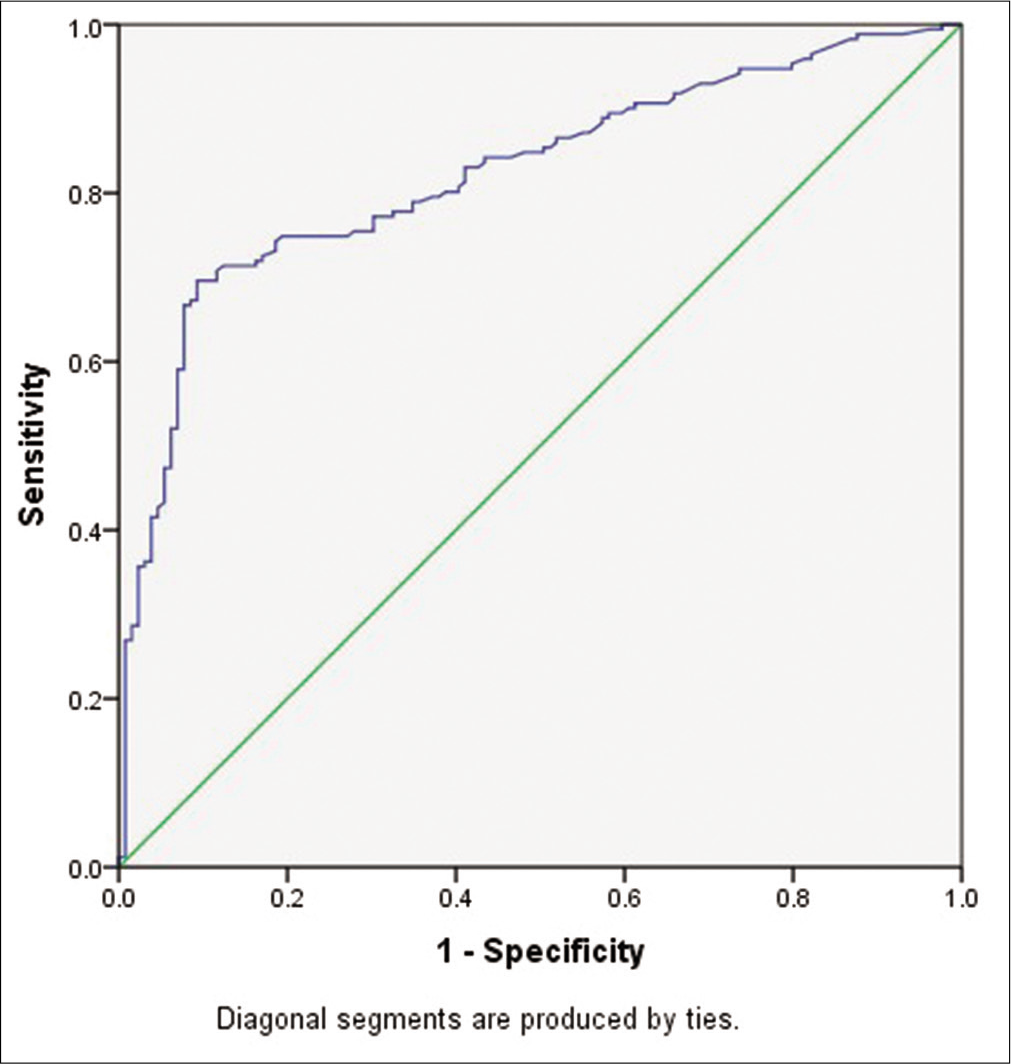
- Receiver operating characteristic curve.
DISCUSSION
In early (placental) and late-onset (maternal) PE, the pathophysiology differs. Based on the pathophysiology of PE, in early pregnancy, there will be abnormal placentation by which there will be a failure in maternal spiral artery transformation which leads to oxidative stress and ends with placental ischemia (Stage I). The ischemic placenta secretes soluble factors like sFlt1 during the third trimester which, in turn, induces placental perfusion, systemic endothelial dysfunction, and PE (Stage II).[6]
In an animal designed (pregnant rats or mice) studies, it is evident that increased levels of sFlt1 are due to overexpression of sFlt1 in mRNA. Due to this, there was a hypoxic environment, and the placenta released hypoxia-inducible Factor 1. This prevents the discharge of VEGF and increased the production of nitric oxide. This hints at vasoconstriction in vessels. The sFlt1 levels were raised before 5 weeks onset of PE clinical conditions and associated with the severity of the disease.[2,9] In Albania, a study was conducted on 95 PE women plasma whose sFlt1 levels are highly significant.[10] By Tang et al., on 105 PE subjects, a study was piloted, they found a sound increase in sFlt1 levels in PE and complicated PE pregnancies, especially in intrauterine fetal growth and development.[11] In a cohort study, in California on 97 pregnant women tested sFlt1 levels by comparing nulliparous and multiparous women, in first and second pregnancies and observed an increase in their sFlt1 levels during first pregnancies than second pregnancy and can be a predictor marker in primigravida. As it is a known factor that primigravida women were at risk for PE. This matches with the present study.[12] The sFlt1 levels were increased with increased maternal age, early gestational weeks, obesity, and in vitro fertilization (IVF). In the present study also the sFlt1 levels are high in late gestational weeks but not affected by maternal age.[13] Along with maternal age, early gestational weeks, obesity, IVF like risk factors, even ethnicity also plays a role. The sFlt1 levels and PE were also associated with ethnicities. There are studies worked on Hispanic women, Caucasian women, and African-Caribbean pregnant women. In Caucasian PE women, sFlt1 levels were elevated significantly when compared with Hispanic PE women.[14] Another study was performed by Lai on African-Caribbean and Caucasian populations, in the African-Caribbean population; the possibility for occurrence of PE is higher with increased sFlt1 levels in serum than Caucasian PE women[13] so based on this the ethnicity also a risk factor in PE. But regarding abnormal implantation of the placenta and excess sFlt1 production is left over as hypothetical. The mean serum sFlt1 value of 7.6 ng/ml in humans was almost 5 times risk in severe PE than in normotensive pregnant women. If sFlt1 overexpression occurs early in pregnancy, it might serve as a diagnostic marker in patients at high risk due to PE.[4] By exhaustive research states that sFlt1 cannot be a predictive diagnostic marker in the 1st trimester of PE pregnancy.[14] Fan et al. in human and animal model studies proved that placental trophoblast cells overexpress sFlt1 in self-defense against excessive VEGFA. This study ensures that increased placental expression of sFlt1, the maternal decidual cells are responsible.[15] In 20 PE subjects studied by Chielie et al., the sFlt1 levels are significantly increased with mean ranges from 6.7 ng/ml to 1.6 ng/ml whereas in controls it ranges from 3.0 ng/ml to 0.95 ng/ml.[3] In an animal (mouse) designed study, by the administration of sFlt1 leads to cardiac dysfunction. This proves that there was a significant association between PE and sFlt1 levels to cardiac dysfunction. There was a much stronger relationship between early-onset PE and later-life cardiovascular diseases in their first pregnancies. Increased sFlt1 levels are correlated with acute myocardial infarction even after postnatal delivery from 4–6 weeks to 5–8 years. The history of PE is a predictable cardiovascular marker in later life.[14] Out of few studies in India especially from the south part, this is the first study. From North India, a total of 6 studies were performed on the sFlt1 levels and PE. From Lucknow, a study was focused on maternal sera sFlt1 levels, which were higher in PE women.[16] In a study of the Delhi population the maternal serum levels of sFlt1 in the PE subjects mean ranged from 6785.25 ± 1677.01 pg/ml and in controls were 3030.29 ± 956.35 pg/ml (mean ± standard deviation). The maternal serum levels of sFlt1 were higher in PE Women.[3] A twin study from the Delhi population evaluated the serum levels with median ranged from 2932.81; 1802.33–5760.46 pg/ml PE against controls with 1114.94; 655.03–2694.35 and found increased sFlt1 levels in PE women along with the association of sFlt1gene polymorphisms.[17,18] From West Bengal-North India, a study was evaluated and found increased serum sFlt1 levels in PE compared to the control group.[19] Another twin study from New Delhi was evaluated on 80 pregnant women and their sFlt1 levels the results were found significantly higher with median 11295.25 pg/ml versus 2936.2 pg/ml in PE than those in the sera of normotensive median 2893.20 pg/ ml, range 1180.43–6706.6 pg/ml.[20] In the present study, we found a significant increase in the sFlt1 levels in PE women compared to the control group with a median ± standard error was 1535.1 ± 237.5 and in the control group was 320 ± 80.2. Hence, this study matches with the above in increased levels of serum sFlt1 in PE women.
The risk factors such as maternal characteristics like maternal age, nulliparity, pre-existing medical conditions, history of PE, and family history of cardiovascular disease, can predict 30% of PE women.[2,5] The other predictors such as uterine artery Doppler scan, angiogenic markers such as soluble endoglin, PIGF, and genetic markers, unknown environmental hazards such as high altitudes, O+ blood group may be responsible in the progress of PE but unfortunately, none of them are focused markers.[5] However, none of the studies have confirmed the sFlt1 or any other markers can be a predictive or diagnostic marker in PE. Along with biophysical markers, biochemical serum, genetic markers individually or in combination cannot predict PE adequately. With the combination of markers, the prediction rate maybe 30%.[21] By various studies from literature, it was proved that sFlt1 alone is competent for vascular endothelial dysfunction/damage.[22,23] Top of form bottom of form endothelial dysfunction is one of the cardinal features in PE. This encourages the use of sFlt1 levels in serum to diagnose the PE condition but still, cohort studies have to be done to predict the disease condition and to know it can be an early diagnostic marker. In the future studies on drug intervention for restoring of sFlt1 should be focused so prolong of PE pregnancy can be planned and also can reduce the endothelial impairment.[5]
CONCLUSION
With this, we conclude that
This is the first South Indian study with a huge sample size of 300 subjects.
The maternal serum concentration of sFlt1 levels in normotensive pregnant women is low compared to preeclamptic women.
If we compare the sFlt1 levels in early and late gestational weeks, in late gestational weeks in controls and PE the levels were highly significant than early gestational weeks of PE and controls.
Hence, sFlt1 levels can be used as a diagnostic marker in the third trimester for PE in the Kolar population.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
References
- Placental hypoxia, endoplasmic reticulum stress and maternal endothelial sensitisation by sFLT1 in preeclampsia. J Reprod Immunol. 2016;114:81-5.
- [CrossRef] [PubMed] [Google Scholar]
- Preeclampsia: Novel mechanisms and potential therapeutic approaches. Front Physiol. 2018;9:973.
- [CrossRef] [PubMed] [Google Scholar]
- Estimation of serum levels of VEGF and SVEGFR-1 (sFLT-1) in preeclampsia. J Evol Med Dent Sci. 2020;9:913-8.
- [CrossRef] [Google Scholar]
- Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Investig. 2003;111:649-58.
- [CrossRef] [PubMed] [Google Scholar]
- Preeclampsia: A renal perspective. Kidney Int. 2005;67:2101-13.
- [CrossRef] [PubMed] [Google Scholar]
- Circulating angiogenic factors and the risk of adverse outcomes among haitian women with preeclampsia. PLoS One. 2015;10:e0126815.
- [CrossRef] [PubMed] [Google Scholar]
- Preeclampsia and soluble fms-like tyrosine kinase 1. J Clin Endocrinol Metab. 2009;94:2252-4.
- [CrossRef] [PubMed] [Google Scholar]
- New laboratory biomarkers for early diagnosis of preeclampsia. Int J Sci Res. 2015;4:1667-9.
- [Google Scholar]
- VEGF and sFLT-1 in serum of PIH patients and effects on the foetus. Exp Ther Med. 2019;17:2123-8.
- [CrossRef] [PubMed] [Google Scholar]
- Circulating levels of the antiangiogenic marker sFLT-1 are increased in first versus second pregnancies. Am J Obstet Gynecol. 2005;193:16-22.
- [CrossRef] [PubMed] [Google Scholar]
- Competing risks model in screening for preeclampsia by serum placental growth factor and soluble fms-like tyrosine kinase-1 at 30-33 weeks' gestation. Fetal Diagn Ther. 2014;35:240-8.
- [CrossRef] [PubMed] [Google Scholar]
- Preeclampsia: Maternal systemic vascular disorder caused by generalized endothelial dysfunction due to placental antiangiogenic factors. Int J Mol Sci. 2019;20:1-18.
- [CrossRef] [PubMed] [Google Scholar]
- sFLT1 in preeclampsia: Trophoblast defense against a decidual VEGFA barrage? J Clin Invest. 2014;124:4690-2.
- [CrossRef] [PubMed] [Google Scholar]
- Serum levels of soluble fms like tyrosine kinase-1(sFlt-1) in normotensive and preeclamptic pregnancy. Int J Med Res Rev. 2013;1:114-9.
- [CrossRef] [Google Scholar]
- Association of vascular endothelial growth factor and soluble fms-like tyrosine kinase-1 polymorphisms with their circulating protein levels in preeclampsia. J Anat Soc India. 2019;68:215-20.
- [CrossRef] [Google Scholar]
- Role of sVEGFR (sFlt-1) in inducing endoplasmic reticulum stress in trophoblast cells and its status in preeclampsia. J Anat Soc India. 2018;67:93-103.
- [CrossRef] [Google Scholar]
- A comparative study of novel biomarkers on preeclampsia in relation to body mass index. Int J Res Pharm Sci. 2020;11:913-20.
- [CrossRef] [Google Scholar]
- Circulating angiogenic factors in pregnancies complicated by pre-eclampsia. Natl Med J India. 2010;23:77-81.
- [Google Scholar]
- Review: Biochemical markers to predict preeclampsia. Placenta. 2012;33:S42-7.
- [CrossRef] [PubMed] [Google Scholar]
- Update on the pathophysiological implications and clinical role of angiogenic factors in pregnancy. Fetal Diagn Ther. 2015;37:81-92.
- [CrossRef] [PubMed] [Google Scholar]
- Placental-specific sFLT-1: Role in pre-eclamptic pathophysiology and its translational possibilities for clinical prediction and diagnosis. Mol Hum Reprod. 2017;23:69-78.
- [CrossRef] [PubMed] [Google Scholar]






