Translate this page into:
Impact of COVID-19 vaccination in patients with auto-immune diseases – A nationwide survey from 842 autoimmune patients
*Corresponding author: Madhan Jeyaraman, Department of Orthopaedics, ACS Medical College and Hospital, Dr. MGR Educational and Research Institute, Chennai, Tamil Nadu, India. madhanjeyaraman@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Muthu S, Manohar M, Selvaraj P, Jeyaraman N, Jeyaraman M, Samagani A, et al. Impact of COVID-19 vaccination in patients with auto-immune diseases – A nationwide survey from 842 autoimmune patients. Indian J Med Sci 2023;75:114-20.
Abstract
Objectives:
To combat the Coronavirus Disease 2019 (COVID-19) pandemic, the World Health Organization announced the emergency license for the usage of COVID-19 vaccinations. Various literature postulated a few cross-talks between autoimmune disease and COVID-19 vaccination. The molecular mimicry between autoimmune diseases as well as autoimmune antibodies and the antibodies against Severe Acute Respiratory Syndrome Coronavirus-2 S proteins triggers the development of a severe form of autoimmune disease. The causal association between autoimmune disease and COVID-19 vaccinations is still under debate. Hence, in this study, we aim to analyze the impact of COVID-19 vaccination on patients with autoimmune diseases.
Material and Methods:
Patients were recruited from a nationwide survey throughout India from October 1, 2021, to December 30, 2021. All patients of autoimmune diseases enrolled in this study had received a diagnosis of COVID-19. A Google form was created in the English language with relevant items, including demographic variables, COVID-19 vaccination-related variables, and its impact on autoimmune disease. Association between the COVID-19 severity, vaccination status, and autoimmune disease status was analyzed.
Results:
Eight hundred and forty-two patients with autoimmune disease participated in the study with 86% of vaccination rate. We noted comparable infection rates among vaccinated (37.5%, n = 272) and non-vaccinated (33.3%, n = 39) respondents with autoimmune disease (P = 0.38). Although 22.5% (n = 163) of patients with autoimmune disease demonstrated deterioration following vaccination, 75.3% (n = 546) of patients did not show any change in disease profile. We noted a significant increase in the computed tomography (CT) severity score of COVID-19 infection among non-vaccinated individuals (odds ratio = 1.1,95% confidence interval [0.29, 2.29], P < 0.001). Moreover, we also noted a significant increase in the need (P = 0.01) and length of hospitalization (P < 0.001) among COVID-19 non-vaccinated individuals. We also noted vaccination significantly prevented an acute flare-up of auto-immune disease when infected with COVID-19 (P < 0.001).
Conclusion:
Although vaccination did not affect the incidence of disease among patients with auto-immune disease, it did significantly decrease the CT severity score, hospitalization rate, and length of stay following COVID-19 infection. Moreover, vaccination also prevented acute flare-ups of autoimmune disease following COVID-19 infection.
Keywords
COVID-19
Autoimmune
Vaccination
Molecular mimicry
INTRODUCTION
Coronavirus disease 2019 (COVID-19) pandemic has created a major challenge in identifying the symptoms, signs, management, and complications .[1,2] The pandemic caused by SARS-CoV-2 affects the morbidity, mortality, and quality of life of the population of various groups.[3] Despite being a respiratory pathogen, Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) had demonstrated extrapulmonary manifestations, making the disease an international public health concern.[4] To combat this COVID-19 disease, the World Health Organization (WHO) announced the emergency license for the usage of COVID-19 vaccinations (Covaxin, Covishield, AstraZeneca/Oxford, Johnson and Johnson, Moderna, Pfizer, Sinopharm, Sinovac, Nuvaxovid, and CanSino)[5] Globally, 494 crores (63.3%) population are fully vaccinated whereas 94.6 crores (68.6%) population are completely vaccinated against COVID-19 disease as of September 18, 2022.[6]
Various literature postulated a few cross-talks between autoimmune disease and COVID-19 vaccination.[7,8] The molecular mimicry between autoimmune diseases as well as autoimmune antibodies and antibodies against SARSCoV-2 S proteins trigger the development of a severe form of autoimmune disease.[9-11] Due to antibody-dependent enhancement mechanisms, the development of vaccine-induced antibodies was observed in autoimmune disease individuals. These vaccine-induced antibodies facilitate antigen-antibody reaction which results in immune complex deposition and tissue damage.[12,13,14] Literature evidence states the lesser COVID-19 infectivity and severity among autoimmune patients in the post-vaccination phase.[7,13] There is no evidence to support the timing of immunomodulatory therapies for inflammatory disorders concerning COVID-19 vaccine safety and efficacy. However, several guidelines have been provided to give medication to optimize vaccine response.[15,16] A recent meta-analysis demonstrated lower vaccine efficacy (70.4%) in immunocompromised patients with solid organ transplants, inflammatory and autoimmune diseases, and malignancy when compared with controls.[17] The incidence of exacerbation in autoimmune disease after COVID-19 vaccination has not significantly differed by the COVID-19 vaccine manufacturer. COVID-19 vaccines were found to be more immunogenic resulting in spike protein-specific T-cell responses and neutralizing antibody levels.[15] Vaccine-induced autoimmune reaction exacerbations are due to the agonists of toll-like receptors (TLRs) – 7, −8, and −9.[18] The stimulation of innate immunity through TLRs induced by mRNAs triggers autoimmune inflammatory diseases.[19] The spatial and temporal association between autoimmune disease and COVID-19 vaccinations is still under debate. Hence, in this study, we aim to analyze the impact of COVID-19 vaccination in patients with autoimmune diseases.
MATERIAL AND METHODS
As a cross-sectional study, we recruited the patients through a nationwide survey throughout India from October, 2021, to December 30, 2021, after obtaining institutional ethical clearance from JJM Medical College (JJMMC/ IEC/2021/00142) on September 20, 2021. All patients with autoimmune diseases enrolled in this study had received a diagnosis of COVID-19 according to the diagnostic criteria from the fifth edition of the Guidelines on the Diagnosis and Treatment of COVID-19 by the National Health Commission of China. The Institutional Ethics Committee, approval was obtained, and written informed consent was received from all the participants. The respondents with autoimmune disease were approached through the snowball technique.
A Google form was created in the English language with relevant items, including demographic variables, COVID-19 vaccination-related variables, COVID-19 severity, and status of autoimmune disease. Comorbidities such as presence of diabetes and hypertension were assessed among the participants. The Google form included a basic description of the purpose for which this survey was conducted. The Google form link, as well as the study’s description and objective, were sent to potential volunteers by mail or social media platforms and the respondents were given a choice to not take part in the study after detailing the study objectives.
Descriptive statistics were reported as mean (standard deviation) for continuous variables, and frequencies (percentage) for categorical variables. Chi-square at a 5% level of significance was used to find statistical significance. Fisher’s exact test is used when the expected cell count is <5. Logistic regression was subjected to finding the relationship between the dependent variable and one or more independent variables. Logistic regression was applied on Co-RADS, computed tomography (CT) severity score, hospitalization, length of hospitalization, and autoimmune ailment during COVID-19 infection with COVID-19 vaccination status. Data were statistically evaluated with IBM SPSS Statistics for Windows, Version 25.0., IBM Corp., Chicago, IL.
RESULTS
There were about 907 total responses of which 899 agreed to take part in the study. Among them, 842 had autoimmune disease, and of them, only 725 were vaccinated. Among them, only 311 had COVID-19 infection [Figure 1].
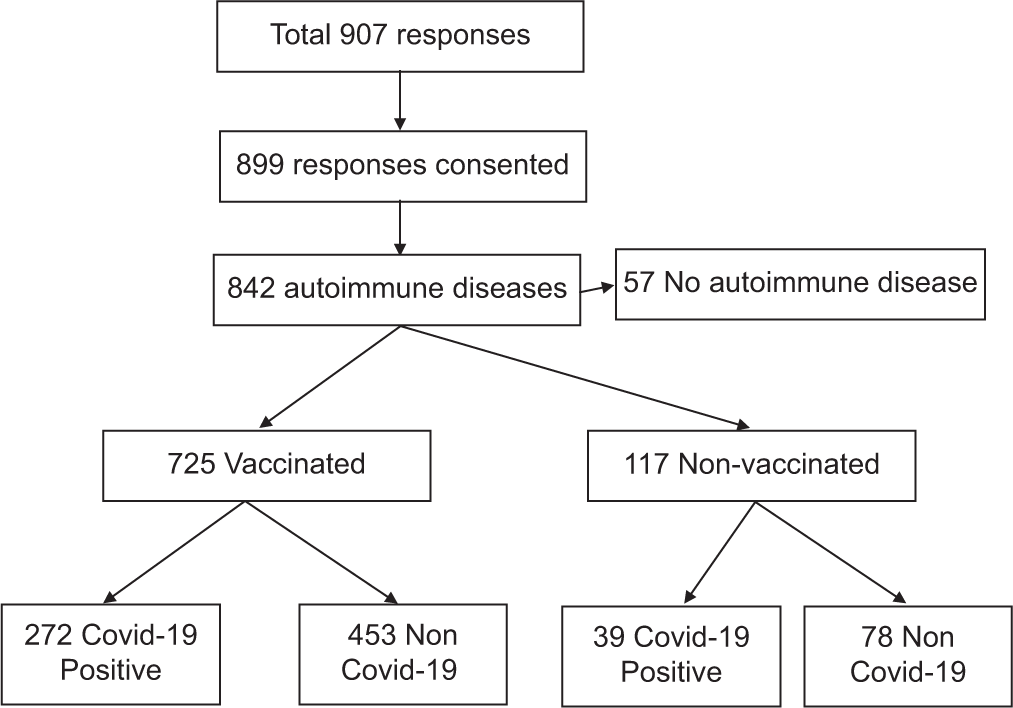
- Flow diagram of the total responses (n=899).
The majority of them were in the age group of 35–44 years (28.6%) followed by 45–54 years (23.2%), 25–34 years (18.7%), 55–64 years (11.3%), 18–24 years (18.7%), and 65 years and above (3.9%). The majority of them were females (52.7%). Around 32.6% had a bachelor’s degree followed by 25.4% being high school graduates, 24.1% uneducated, 15.1% master’s degree, and 2.8% with doctorate degrees. The majority of the respondents were from South India (47.5%), followed by North India (17.7%) and Central India (11.9%) as shown in [Table 1].
| Variable | Frequency | Percentage |
|---|---|---|
| Age | ||
| 18–24 | 128 | 14.2 |
| 25–34 | 168 | 18.7 |
| 35–44 | 257 | 28.6 |
| 45–54 | 209 | 23.2 |
| 55–64 | 102 | 11.3 |
| 65 and above | 35 | 3.9 |
| Gender | ||
| Male | 425 | 47.3 |
| Female | 474 | 52.7 |
| Education | ||
| Bachelor’s degree | 293 | 32.6 |
| Doctorate degree | 25 | 2.8 |
| High school graduate | 228 | 25.4 |
| Master’s degree | 136 | 15.1 |
| None of the above | 217 | 24.1 |
| Region | ||
| Central India | 107 | 11.9 |
| East India | 73 | 8.1 |
| North India | 159 | 17.7 |
| Northeast India | 77 | 8.6 |
| Others | 9 | 1.0 |
| South India | 427 | 47.5 |
| Western India | 47 | 5.2 |
[Table 2] gives the distribution of the spectrum of autoimmune diseases included in the study. Rheumatoid arthritis (16.7%) and psoriasis (15.7%) were the major contributors. Around 1.2% of the respondents with the autoimmune disease had more than one autoimmune disease. Around 37.5% of the respondents with the autoimmune disease had mild severity, 51.5% moderate severity, 10.5% severe, and 0.5% very severe autoimmune disease. Around 53.7% of the respondents with the autoimmune disease were in an active phase of illness, 28.5% inactive phase, 14.7% were in the remission phase, and 3.1% were in a disease flare-up following remission. Around 38.5% of the respondents with the autoimmune disease had comorbidities, 1% had a history of an allergic reaction, and 1.1% had a history of immunoglobulin therapy or blood product transfusion. Among the respondents with autoimmune disease, 86.1% had been vaccinated for COVID-19.
| Variable | Frequency | Percentage |
|---|---|---|
| Rheumatoid arthritis | 141 | 16.7 |
| Psoriasis | 132 | 15.7 |
| Antiphospholipid syndrome | 80 | 9.5 |
| Vitiligo | 64 | 7.6 |
| Polymyositis | 46 | 5.5 |
| Myasthenia gravis | 45 | 5.3 |
| Alopecia areata | 40 | 4.8 |
| Psoriatic arthritis | 23 | 2.7 |
| Ankylosing spondylitis | 21 | 2.5 |
| Reactive arthritis | 21 | 2.5 |
| Inflammatory bowel disease | 20 | 2.4 |
| Systemic lupus erythematosus | 18 | 2.1 |
| Pemphigus vulgaris | 16 | 1.9 |
| Sarcoidosis | 15 | 1.8 |
| Others | 160 | 19.0 |
| More than one autoimmune disease | 10 | 1.2 |
| Severity | ||
| Mild | 316 | 37.5 |
| Moderate | 434 | 51.5 |
| Severe | 88 | 10.5 |
| Very severe | 4 | 0.5 |
| Current phase of disease | ||
| Active phase | 452 | 53.7 |
| Flare following remission | 26 | 3.1 |
| Inactive phase | 240 | 28.5 |
| Remission phase | 124 | 14.7 |
| Comorbidities | ||
| Present | 324 | 38.5 |
| Absent | 518 | 61.5 |
| History of allergic reaction to any licensed or unlicensed vaccine? | ||
| Present | 8 | 1.0 |
| Absent | 834 | 99.0 |
| History of immunoglobulin therapy or blood products transfusion within the past month? | ||
| Present | 9 | 1.1 |
| Absent | 832 | 98.9 |
| Vaccinated | ||
| Yes | 725 | 86.1 |
| No | 117 | 13.9 |
Among the autoimmune vaccinated respondents (n = 725), around 43.6% had CoviShield and 41.9% Covaxin [Table 3]. About 73.7% of them were fully vaccinated. Around 46.9% had an interval of 4–6 weeks between the two doses of the vaccine while 18.1% had an interval of 6–8 weeks between two doses, 7% had an interval of 8–12 weeks and 1.7% had an interval of more than 12 weeks between the vaccine doses. Although 75.3% of the patient did not note any change in their disease status following vaccination, 22.5% had sudden deterioration due to flare-up of autoimmune disease post-vaccination, 1.2% had deterioration requiring hospitalization, and 1.0% had deterioration requiring intensive care unit care. Moreover, 6.3% reported adverse events following the vaccination with musculoskeletal symptoms.
| Variable | Frequency | Percentage |
|---|---|---|
| Type of vaccine | ||
| Covaxin | 353 | 41.9 |
| CoviShield | 367 | 43.6 |
| Others | 4 | 0.5 |
| None | 1 | 0.1 |
| History of any other vaccine intake prior to any dose of COVID-19 vaccine (either 2 weeks prior or until 30 days) | ||
| Present | 31 | 4.27 |
| Absent | 694 | 95.73 |
| Fully vaccinated | ||
| Yes | 534 | 73.7 |
| No | 191 | 26.3 |
| Interval between two doses of vaccine | ||
| 4–6 weeks | 340 | 46.9 |
| 6–8 weeks | 131 | 18.1 |
| 8–12 weeks | 51 | 7.0 |
| More than 12 weeks | 12 | 1.7 |
| Any change in autoimmune disease post vaccination |
||
| Deterioration requiring hospitalization | 9 | 1.2 |
| Deterioration requiring intensive care unit care | 7 | 1.0 |
| Sudden deterioration | 163 | 22.5 |
| No change | 546 | 75.3 |
| Reported any adverse event following vaccine |
||
| Yes | 46 | 6.3 |
| No | 679 | 93.7 |
| Severity | ||
| Mild | 96 | 30.9 |
| Moderate | 188 | 60.5 |
| Severe | 27 | 8.7 |
Among vaccinated respondents with autoimmune disease, 37.5% were COVID-19 positive. Similarly, 33.3% of nonvaccinated respondents with autoimmune disease were also infected with COVID-19 infection. Among the autoimmune vaccinated respondents, 30.9% had a mild COVID-19 infection, 60.5% had a moderate COVID-19 infection, and only 8.7% had a severe COVID-19 infection as shown in [Table 4]. However, a significant proportion of the population in both groups was not aware of their clinical severity.
| Variable | COVID-19 vaccinated (n=725) | Non- vaccinated (n=117) | χ2(df), P |
|---|---|---|---|
| COVID-19-positive | |||
| Yes | 272 (37.5) | 39 (33.3) | 0.76 (1), 0.38 |
| No | 453 (62.5) | 78 (66.7) | |
| Severity | |||
| Mild | 80 (11) | 16 (13.7) | |
| Moderate | 171 (23.6) | 17 (14.5) | |
| Severe | 21 (2.9) | 6 (5.1) | 6.161 (3), 0.10 |
| Not aware | 453 (62.5) | 78 (66.7) |
On analyzing the severity scores of the two groups, the CT severity score was 1.10 times more among COVID-19 nonvaccinated individuals (P < 0.001) [Table 5]. Moreover, we noted a significant proportion of non-vaccinated patients required hospitalization following COVID-19 infection (P = 0.01) and we also noted that the length of hospitalization was significantly longer among COVID-19 non-vaccinated individuals compared to vaccinated individuals (P < 0.001). We also noted vaccination significantly prevented an acute flare-up of auto-immune disease when infected with COVID-19 (P < 0.001).
| Variable | Non vaccinated (n=39) | COVID-19 vaccinated (n=272) | χ2(df), P | OR (95% CI) |
|---|---|---|---|---|
| CO-RADS | ||||
| CO-RADS 1 | 1 (2.6) | 2 (0.7) | 8.164 (6), | - |
| CO-RADS 2 | 3 (7.7) | 7 (2.6) | 0.22 | |
| CO-RADS 3 | 3 (7.7) | 33 (12.1) | ||
| CO-RADS 4 | 3 (7.7) | 16 (5.9) | ||
| CO-RADS 5 | 1 (2.6) | 35 (12.9) | ||
| CO-RADS 6 | 23 (59) | 142 (52.2) | ||
| Not aware | 5 (12.8) | 37 (13.6) | ||
| CT severity score | ||||
| <8 (Mild) | 7 (17.9) | 35 (12.9) | 24.26 (3), | 0.49 (0.17–1.38) |
| 9–15 (Moderate) | 11 (28.2) | 181 (66.5) | <0.001 | 0.15 (0.06–0.36) |
| >15 (Severe) | 8 (20.5) | 24 (8.8) | 1.10 (0.29–2.29) | |
| Not aware | 13 (33.3) | 32 (11.8) | 1 | |
| Hospitalization | ||||
| Required | 30 (76.9) | 206 (75.7) | 6.511 (1), | 1.07 (0.48–2.36) |
| Not Required | 9 (23.1) | 66 (24.3) | 0.01 | 1 |
| Length of hospitalization | ||||
| <1 week | 6 (15.4) | 11 (4) | 25.40 (5), | 2.14 (0.68–6.74) |
| >6 weeks | 0 | 1 (0.4) | <0.001 | - |
| 1–2 weeks | 10 (25.6) | 125 (46) | 0.31 (0.13–0.74) | |
| 2–4 weeks | 6 (15.4) | 75 (27.6) | 0.31 (0.11–0.86) | |
| 4–6 weeks | 2 (5.1) | 1 (0.4) | 7.86 (0.67–92.67) | |
| Not aware | 15 (38.5) | 59 (21.7) | 1 | |
| Autoimmune ailment during COVID-19 infection | ||||
| Decreases in symptoms | 0 | 95 (34.9) | 24.523 (3), | - |
| Gradual increase in symptoms | 4 (10.3) | 49 (18.7) | <0.001 | 0.44 (0.19–1.02) |
| Sudden increase in symptoms | 8 (20.5) | 6 (2.2) | 2.76 (0.72–10.48) | |
| No Change | 27 (69.2) | 122 (44.2) | 1 |
OR: Odds ratio, CI: Confidence interval
DISCUSSION
Even if only adults are vaccinated, a vaccine with a 95% efficacy against disease could significantly reduce future attack rates, hospitalizations, and deaths. Even as vaccines become more widely available over time, non-pharmaceutical interventions continue to play an important role in containing outbreaks. Hospitalization and mortality rates from COVID-19 are significantly higher among adults under 65 who have not been vaccinated than among adults who have received the primary series of the vaccine and who are up to date with subsequent doses.[20] Tens of millions of lives have been saved around the world thanks to the vaccination against COVID-19. However, the impact in low-income settings has been limited due to a lack of access to vaccines, highlighting the importance of global vaccine equity and coverage.
There are those who, due to illness or its treatment, have an impaired immune system and are therefore considered immunocompromised. Patients undergoing chemotherapy for cancer treatment or those who have received a solid organ transplant such as a kidney or heart and are taking medications to maintain their new organ are examples. Some people have to take medicines, such as corticosteroids, that suppress the immune system for an extended period. Such extended use raises the risk of developing secondary or acquired immunodeficiency.
Some individuals who are moderately or severely immunocompromised should receive an additional primary dose and a booster dose after completing the primary vaccination series.[21] Specific recommendations have been crafted because the immune response to the COVID-19 vaccine may vary between people with moderate and severe immunosuppression. Newer COVID-19 vaccines offer better protection against emerging strains, and the centers for disease control and prevention (CDC) recommends that everyone 12 and older get a booster shot. In addition to the original SARS-CoV-2 strain, the most recent Omicron subvariants, BA.4 and BA.5 are the targets of updated boosters, also known as bivalent boosters.[22]
Among 842 participants with autoimmune disease, only 725 were vaccinated. Of which only 311 suffered COVID-19 infection. The occurrence of COVID-19 infection and severity were similar among vaccinated and non-vaccinated respondents with autoimmune disease. Autoimmune diseases are diverse conditions that are connected to an immune system that is not functioning properly. The majority of patients who suffer from autoimmune diseases have either been treated in the past with immunomodulatory medications or biological agents or are currently being treated with such treatments. Patients with autoimmune diseases reduced the number of times that they went to the doctor during the pandemic of COVID-19 because they were worried about the immunosuppressive effects of medications and the contagious nature of SARS-CoV-2. On the other hand, rheumatologic disease flares and worsened disease activity are associated with disruptions in the continuity of medical care as well as non-adherence to prescribed medications. Building a dependable telemedicine platform and providing education on the importance of taking medications as prescribed are therefore highly recommended. The sudden deterioration in the disease status following vaccination noted in 22.5% of the vaccinated population might be explained due to the prior exposure to COVID-19 infection which might have resulted in a robust immune response following the first dose of vaccination along with an increased exacerbation of the autoimmune disease.[15,23]
Since the beginning of this pandemic, infection risk in patients with autoimmune diseases has been a subject of interest.[24-26] Association between autoimmune diseases and COVID-19 as assessed in both a test-negative case–control and population case–control design. Patients suffering from autoimmune diseases had a rate of COVID-19 infection that was comparable to that of the general population, according to the findings of a cross-sectional study that was carried out in the northeastern region of Italy.[25] Another study carried out in Italy, this time in Milan, confirmed that having an autoimmune disease does not increase one’s likelihood of testing positive for COVID-19.[24] We also noted in our study that vaccination did not affect the susceptibility to infection disregarding their vaccination status.
On the other hand, the findings of a retrospective multicentric study that was carried out in Hubei, China, indicated that patients with autoimmune diseases might be more susceptible to COVID-19 infection when compared with controls.[27] In addition to that, this research looked at the patients’ relatives who lived in the same area during the outbreak and used them as a control group to investigate.[24] An interesting finding from a study conducted in Milan was that patients suffering from autoimmune diseases did not have a worse prognosis compared to individuals who did not suffer from autoimmune diseases. On the other hand, the findings of a study conducted in Spain showed that patients hospitalized with autoimmune diseases had a more severe course of COVID-19.[28] Based on the results of our study, we could demonstrate that patients with autoimmune diseases who were vaccinated did not demonstrate a severe course of disease or hospitalization compared to non-vaccinated individuals.
One interesting finding from our study is that 34.9% of vaccinated patients noted a decrease in the symptoms of auto-immune disease with COVID-19 infection which needs further exploration. One possible explanation is that the severity of the COVID-19 infection might have masked the patient from perceiving the severity of the autoimmune disease. We also demonstrated that vaccination prevented these patients from getting an acute flare in their disease status following COVID-19 infection.
Our study has certain limitations. Recall bias is an intrinsic limitation of any cross-sectional study and our study is not an exception as noted in the results with some sections recording “unaware” as the patient response. The snowball technique could be useful for studying very rare diseases, but its main drawback is the high level of sampling bias. There might also be a temporal correlation between the disease flares to the COVID-19 vaccine which might have been purely coincidental and the vaccine might not have been an autoimmune trigger. Due to the design of the study, the most severe cases of COVID-19, those that died, could not have been accessed and could not have responded to the questionnaire. Therefore, the most solid data, regarding the effect of the vaccines on rheumatic disease patient survival cannot be addressed. Study focuses on the patient’s perception of the effects of the vaccine, not the nocebo effect, which is well-known to prevail among patients with autoimmune conditions. The study was conducted in India and recognized the importance of considering the geographical and cultural context when interpreting the results.
CONCLUSION
Although vaccination did not affect the incidence of disease among patients with auto-immune disease, it did significantly decrease the CT severity score, hospitalization rate, and length of stay following COVID-19 infection. Moreover, vaccination also prevented acute flare-ups of autoimmune disease following COVID-19 infection.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Coronavirus disease 2019 (COVID-19): A literature review. J Infect Public Health. 2020;13:667-73.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA. 2020;324:782-93.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of Covid-19 on health-related quality of life of patients: A structured review. PLoS One. 2021;16:e0259164.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19: An international public health concern. Cent Asian J Glob Health. 2020;9:e466.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 Vaccines. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines [Last accessed on 2022 Sep 18]
- [Google Scholar]
- Boost COVID-19 Vaccination Coverage. Geneva: WHO; Available from: https://www.who.int/southeastasia/news/detail/01-07-2022-boost-covid-19-vaccination-coverage--who [Last accessed on 2022 Sep 18]
- [Google Scholar]
- SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol Int. 2021;41:509-18.
- [CrossRef] [PubMed] [Google Scholar]
- Adaptive immunity and the risk of autoreactivity in COVID-19. Int J Mol Sci. 2021;22:8965.
- [CrossRef] [PubMed] [Google Scholar]
- Multisystem inflammatory syndrome and autoimmune diseases following COVID-19: Molecular mechanisms and therapeutic opportunities. Front Mol Biosci. 2022;9:804109.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 and autoimmune diseases. Curr Opin Rheumatol. 2021;33:155-62.
- [CrossRef] [PubMed] [Google Scholar]
- The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev. 2021;20:102792.
- [CrossRef] [PubMed] [Google Scholar]
- American college of rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: Version 1. Arthritis Rheumatol. 2021;73:1093-107.
- [CrossRef] [Google Scholar]
- A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 2020;584:353-63.
- [CrossRef] [PubMed] [Google Scholar]
- Autoimmune skin disease exacerbations following COVID-19 vaccination. Front Immunol. 2022;13:899526.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 vaccine safety and efficacy in patients with immune-mediated inflammatory disease: Review of available evidence. J Am Acad Dermatol. 2021;85:1274-84.
- [CrossRef] [PubMed] [Google Scholar]
- Short-term effectiveness of COVID-19 vaccines in immunocompromised patients: A systematic literature review and meta-analysis. J Infect. 2022;84:297-310.
- [CrossRef] [PubMed] [Google Scholar]
- A possible role for anti-idiotype antibodies in SARS-CoV-2 infection and vaccination. N Engl J Med. 2022;386:394-6.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 vaccination can occasionally trigger autoimmune phenomena, probably via inducing age-associated B cells. Int J Rheum Dis. 2022;25:5-6.
- [CrossRef] [PubMed] [Google Scholar]
- Summary of guidance for minimizing the impact of COVID-19 on individual persons, communities, and health care systems-United States, August 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1057-64.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 Vaccination. 2020. United States: Centers for Disease Control and Prevention; Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html [Last accessed on 2022 Sep 18]
- [Google Scholar]
- Adapted Vaccine Targeting BA.4 and BA.5 Omicron Variants Original SARS-CoV-2 Recommended for Approval. 2022. Netherlands: European Medicines Agency; Available from: https://www.ema.europa.eu/en/news/adapted-vaccine-targeting-ba4-ba5-omicron-variants-original-sars-cov-2-recommended-approval [Last accessed on 2022 Sep 18]
- [Google Scholar]
- Robust antibody responses to the BNT162b2 mRNA vaccine occur within a week after the first dose in previously infected individuals and after the second dose in uninfected individuals. Front Immunol. 2021;12:722766.
- [CrossRef] [PubMed] [Google Scholar]
- Association between autoimmune diseases and COVID-19 as assessed in both a test-negative case-control and population case-control design. Auto Immun Highlights. 2020;11:15.
- [CrossRef] [PubMed] [Google Scholar]
- SARS-CoV-2 infection in patients with autoimmune rheumatic diseases in northeast Italy: A cross-sectional study on 916 patients. J Autoimmun. 2020;112:102502.
- [CrossRef] [PubMed] [Google Scholar]
- Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clin Immunol. 2020;215:108410.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 in patients with rheumatic disease in Hubei province, China: A multicentre retrospective observational study. Lancet Rheumatol. 2020;2:e557-64.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: A multicentric matched cohort study. Ann Rheum Dis. 2020;79:1544-9.
- [CrossRef] [PubMed] [Google Scholar]







