Translate this page into:
Anti-transcription intermediary factor 1 gamma antibody-associated paraneoplastic dermatomyositis: A case report
*Corresponding author: Marius Emanuel Dsouza, Department of Internal Medicine, Grant Government Medical College and Sir JJ Group of Hospitals, Mumbai, Maharashtra, India. mariusdsouza@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Dsouza ME, Sakariya M, Supe AR, More D. Anti-transcription intermediary factor 1 gamma antibody-associated paraneoplastic dermatomyositis: A case report. Indian J Med Sci. 2024;76:134-6. doi: 10.25259/IJMS_39_2024
Abstract
Dermatomyositis (DM) is an inflammatory myopathy characterized by distinctive cutaneous manifestations. Recent advancements have led to the identification of a novel myositis-specific autoantibody called anti-transcription intermediary factor 1 gamma (anti-TIF-1-γ) which presents with unique cutaneous manifestations and a heightened risk for malignancy. We report a case of a 55-year-old female who presented with proximal muscle weakness, an erythematous rash over the face, blanching erythema over the nape of the neck, and red-on-white lesions over the chest. She was diagnosed with anti-TIF-1-γ positive DM and found to have a palpable mass in the right breast. Biopsy showed triple negative invasive breast carcinoma no special type (NST) grade 3. She showed poor response to steroids and was referred for cancer staging and treatment. The anti-TIF-1-γ antibody serves as a novel tool to identify a subset of DM patients at high risk for cancer. At present, its role in the prognosis or surveillance of cancer recurrence remains uncertain.
Keywords
Dermatomyositis
Anti-transcription intermediary factor 1 gamma
Cancer
INTRODUCTION
Dermatomyositis (DM), characterized by an inflammatory myopathy and distinctive cutaneous manifestations has garnered increased attention in recent years. Evolving research has led to the identification of novel myositis-specific autoantibodies, including anti-transcription intermediary factor 1 gamma (anti-TIF-1-γ), anti-NXP2, anti-MDA5, anti-SAE, and anti-HMGCR in a subset of patients with myositis. Among these, the TIF-1-γ antibody has emerged with unique cutaneous findings and a strong association with cancer. In this case report, we present a compelling clinical illustration of the skin manifestations associated with TIF-1-γ antibody positive DM, and its heightened association with malignancy.
CASE REPORT
A 55-year-old female presented with a 2-month history of joint pain, fever, weight loss, rash, and proximal muscle weakness. The joint pain initially began in the ankles and progressed to involve the elbows and shoulders, sparing the small joints of the hands and feet. This was accompanied by subjective fevers. Later, she developed facial redness and an erythematous rash on the chest and back. She also developed proximal muscle weakness, characterized by difficulty lifting her arms above her head and rising from a seated position. Grip strength remained intact. She reported difficulty in swallowing and a 4 kg weight loss in the preceding 2 months. Her past medical history included an abdominal hysterectomy of unclear indication and hypertension on standard therapy. On examination, pulse was 82/min, and blood pressure was 130/80 mmHg. Examination of the skin showed facial erythema, blanching erythema over the nape of the neck [Figure 1], and hypopigmented patches interspersed with erythematous macules on the chest forming a “red on white” lesion pattern [Figure 2]. No skin tightening or Raynaud’s phenomenon was noted. Motor examination revealed power of 2/5 in the shoulders and hips, and 4+/5 power distally. Reflexes were intact, and the sensory examination was unremarkable. A hard, round mass measuring 3 × 3 cm was palpable in the upper outer quadrant of the right breast. No axillary lymph nodes were palpable, and joint examination revealed no synovitis or deformity. Investigations showed hemoglobin 10.9 g/dL, white blood cells 6.9 × 10^3/microl, platelets 209 × 10^3/microl, creatinine 0.7 mg/dL, serum bilirubin 0.4 mg/dL, aspartate aminotransferase 56 U/L, alanine aminotransferase 34 U/L erythrocyte sedimentation rate 68 mm/h, creatine kinase 1094 U/L (normal range 30–145 U/L), anti-nuclear antibody 1:320, speckled pattern, Ro 52kD positive, anti-TIF-1-γ-strong positive, anti-Jo-1-negative, anti-U1-RNP-negative, and anti-double stranded DNA-negative. The urine routine was negative for proteinuria. Echocardiography was normal. EMG showed an early recruitment pattern in the proximal muscles suggestive of a myopathy. Nerve conduction velocity was normal. A diagnosis of DM was made based on Bohan and Peter’s criteria. Considering the strong association of the TIF-1-γ antibody with cancer, she was promptly worked up for the breast mass. Breast ultrasound revealed a 2.8 × 3.1 × 2.8 cm heterogeneously hypoechoic lesion in the upper outer quadrant of the right breast with features suggestive of malignancy. An enlarged hypoechoic lymph node measuring 2.9 × 1.8 cm in the right axilla was also identified. Biopsy of the breast lesion showed invasive breast carcinoma NST grade 3 with estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 negative status. The patient showed poor response to high dose methylprednisolone 1 g daily for 5 days. She was referred to a cancer institute for breast cancer staging and treatment.
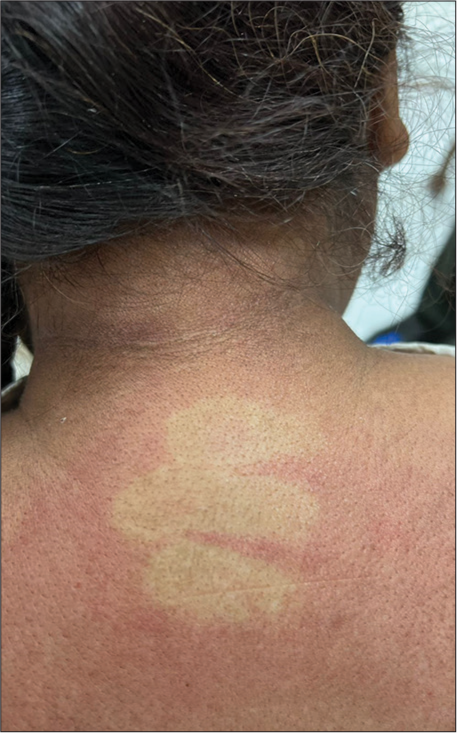
- Blanching erythema over the nape of the neck.
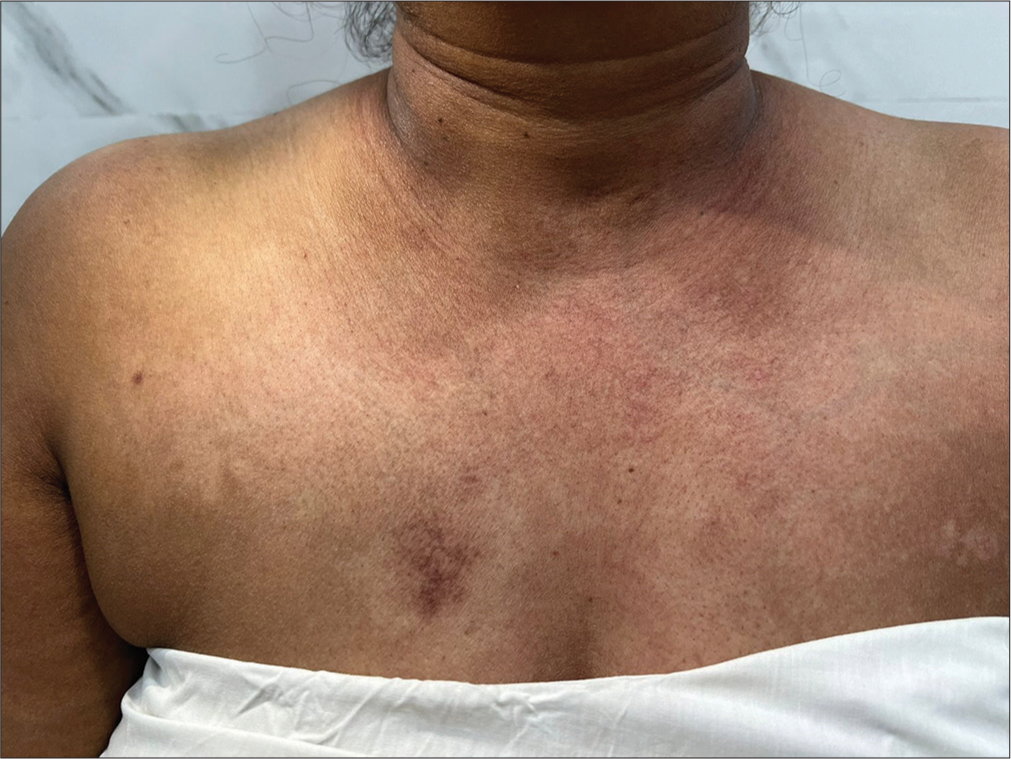
- Hypopigmented patches interspersed with erythematous macules over the chest forming a “red on white” lesion pattern.
DISCUSSION
The TIF-1-γ antibody was initially identified by Kaji et al. as an autoantibody reactive with 155 and 140 kDa nuclear proteins in certain DM patients.[1] Those exhibiting positive titers for anti-155/140 kDa antibodies displayed a higher frequency of skin manifestations compared to their antibody-negative counterparts. Lung involvement in these patients was uniquely absent. Furthermore, a striking 71% of individuals with these antibodies were found to harbor an underlying malignancy, a prevalence significantly higher than the 11% observed in DM patients lacking anti-155/140 kDa antibodies. These antibodies were subsequently recognized to be part of the TIF1 family of homologous proteins.
Since then, we have come to understand the various phenotypical and pathological subtypes of DM associated with different myositis-specific antibodies.[2] Characteristic skin lesions in this antibody group include red-on-white lesions [Figure 2], flagellate erythema, psoriasis-like lesions, hyperkeratotic small papules on the palmar and digital flexor surfaces, and ovoid palatal patch.[3] Our patient also demonstrated blanching erythema on the nape of the neck [Figure 1] which was unique and resembled erythema in patients with dengue fever. Overall, the rash exhibited a photo-exposed pattern. The classical heliotrope rash and Gottron’s papules were notably absent.
The TIF-1-γ antibody has emerged as a robust predictor of malignancy. A 2018 systematic review and meta-analysis demonstrated a substantial odds ratio of 9.37 for cancer-associated DM in antibody-positive patients.[4] This underscores the clinical utility of the TIF-1-γ antibody in identifying a subset of DM patients at heightened risk of cancer. Furthermore, a study by Aussy et al. revealed a 100% positive predictive value for cancer using the anti-TIF-1-γ immunoglobulin G2 isotype at a cutoff >385.[5] Despite the lack of specific guidelines on cancer workup in DM patients, the strong association of anti-TIF-1-γ with malignancy advocates for a detailed physical examination and thorough investigation, including whole-body positron emission tomography-computed tomography, to uncover potential underlying malignancies. Further studies are needed to evaluate the role of antibody titers in monitoring response to chemotherapy and cancer recurrence.
CONCLUSION
Within the diverse spectrum of DM patients, there are distinctive skin manifestations associated with myositis-specific antibodies such as the TIF-1-γ antibody that are clinically discernible. Recognizing them may prompt further myositis-specific antibody testing. Even after securing a diagnosis of DM based on Bohan and Peter’s criteria, testing for the TIF-1-γ antibody proves valuable in identifying individuals at heightened risk for cancer. This recognition should prompt an extensive workup for underlying malignancy to facilitate early cancer detection. The incorporation of TIF-1-γ antibody testing in the diagnostic and prognostic evaluation of DM, therefore, holds considerable clinical significance.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Identification of a novel autoantibody reactive with 155 and 140-kDa nuclear proteins in patients with dermatomyositis: An association with malignancy. Rheumatology (Oxford). 2007;46:25-8.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatomyositis clinical and pathological phenotypes associated with myositis-specific autoantibodies. Curr Rheumatol Rep. 2018;20:28.
- [CrossRef] [PubMed] [Google Scholar]
- Distinctive cutaneous and systemic features associated with antitranscriptional intermediary factor-1g antibodies in adults with dermatomyositis. J Am Acad Dermatol. 2015;72:449-55.
- [CrossRef] [PubMed] [Google Scholar]
- Use of anti-transcriptional intermediary factor-1 gamma autoantibody in identifying adult dermatomyositis patients with cancer: A systematic review and meta-analysis. Acta Derm Venereol. 2019;99:256-62.
- [CrossRef] [PubMed] [Google Scholar]
- The IgG2 isotype of anti-transcription intermediary factor 1g autoantibodies is a biomarker of cancer and mortality in adult dermatomyositis. Arthritis Rheumatol. 2019;71:1360-70.
- [CrossRef] [PubMed] [Google Scholar]






