Translate this page into:
A case of atypical complication of severe leptospirosis as Guillain-Barre syndrome
*Corresponding author: Rahul Soni, Department of Neurology, Base Hospital Delhi Cant, Delhi, India. drrahulsoni1977@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Soni R, Summi P, Debnath J. A case of atypical complication of severe leptospirosis as Guillain-Barre syndrome. Indian J Med Sci. doi: 10.25259/IJMS_122_2024
Abstract
Neurological complications in Leptospirosis are rare, and may manifest early and late also. We present atypical delayed neurological complication of Leptospira as Guillain Barre Syndrome. A 51 year old diabetic presented with high-grade intermittent fever with chills associated with shortness of breath, vomiting and diarrhea, and reduced urinary output. His septic shock and severe sepsis caused lead to acute kidney injury, hepatitis, and left ventricular dysfunction. On investigation Leptospiral IgM antibodies was positive. He was managed with antibiotics, continues reno-replacement therapy, and mechanical ventilation support. Following his recovery from Leptospira-related systemic problems, he developed quadriparesis with truncal weakness and areflexia without sensory involvement. Nerve conduction study suggested polyneuropathy involving bilateral upper and lower limbs with sparing of sural nerve and impersistent F wave. He was diagnosed with Guillain Barre Syndrome and was treated with Immunoglobulins 150 grams over the course of five days. He improved and gained independence in all spheres of life. Leptospirosis is a potentially serious illness, often results in multi-organ failure and necessitates ICU admission. Initial signs and symptoms and routine laboratory investigations can be nonspecific. Because neurological problems are uncommon and often take time to show, it is imperative to monitor these individuals.
Keywords
Leptospira
Guillain-Barre syndrome
Nerve conduction study
INTRODUCTION
Leptospirosis interrogans causes leptospirosis. It can be mild self-limiting to fulminant in the course. Neurological complications are rare and may manifest early and late. We describe the atypical delayed neurological complication of leptospira as Guillain-Barre syndrome (GBS).
CASE REPORT
A 51 years man, known diabetic, presented with high-grade intermittent fever with chills associated with shortness of breath, vomiting, diarrhea, and reduced urinary output. His respiratory rate was 36/min, oxygen saturation was 84% at room air, blood pressure was 86/56 mm Hg, and heart rate was 110/min. Leukocyte count was 30100/cumm with polymorphous 84%, mild anemia (12.2 g/dL), and normal platelet count. Urea and creatinine were 207 mg/dL and 6.30 mg/dL, respectively. Sodium and potassium were 124 mEq/L and 2.4 mEq/L. Total bilirubin was 2.7 mg/dL, and both aspartate transaminase and alanine aminotransferase were 80 IU/L. His procalcitonin level was 5.6 ng/mL. Electrocardiography was normal. However, echocardiography revealed a left ventricular ejection fraction of 35% with moderate mitral regurgitation.
The patient was managed as a case of severe sepsis with septic shock with antibiotics in renal-modified dosages. The patient further deteriorated and had a continuous fever, and leukocytosis further raised to 46200/cumm. His procalcitonin increased to 16.2 ng/mL. His antibiotics were changed as per the hospital antibiotic policy. He became drowsy and developed respiratory fatigue. Hence, he was intubated and supported with mechanical ventilation. Since body fluid cultures were sterile, procalcitonin was increasing gradually and not responding to broad-spectrum antibiotics; he was tested for leptospiral immunoglobulin M (IgM), which turned out to be positive. Antibiotics change to ceftriaxone and tigecycline. Continues reno-replacement therapy (CRRT) equipped with lipopolysaccride absorption cartilage with hemofiltration filter was given for acute kidney injury (AKI). He has shown good improvement, leukocytosis reduced, and AKI recovered. His procalcitonin was decreased to 0.5 ng/mL, clinical features continued to improve, and he was extubated after 5 days of intubation.
After 7 days of extubating, he developed pure motor weakness in all four limbs. Power in both upper limbs power was 2/5, right lower limb was 2/5, and left lower limb was 1/5. He was also not able to raise his head above the pillow and turn on the bed. All his reflexes were absent. There was no respiratory distress. Critical care neuropathy and GBS were considered differential diagnoses. A nerve conduction study (NCS) suggested axonal polyneuropathy involving bilateral upper and lower limb with normal sural nerve conduction and impersistent reduced F wave in the bilateral ulnar and median nerve [Figures 1-3]. Cerebrospinal fluid (CSF) analysis showed protein 52.5 mg/dL, sugar 104 mg/dL, and white blood count was 01/cumm. He was diagnosed with an acute motor axonal neuropathy variant of GBS as per Asbury criteria. He was treated with IV immunoglobulins 150 g over 5 days. Within the next 5 days, gradually, his weakness improved with power 4/5 bilateral upper limbs and 4/5 bilateral lower limbs. A repeat echocardiogram showed an improved left ventricular ejection fraction of 50% with mild global hypokinesia. NCS after four weeks was suggestive of mild improvement in right tibial nerve [Figures 4 and 5].
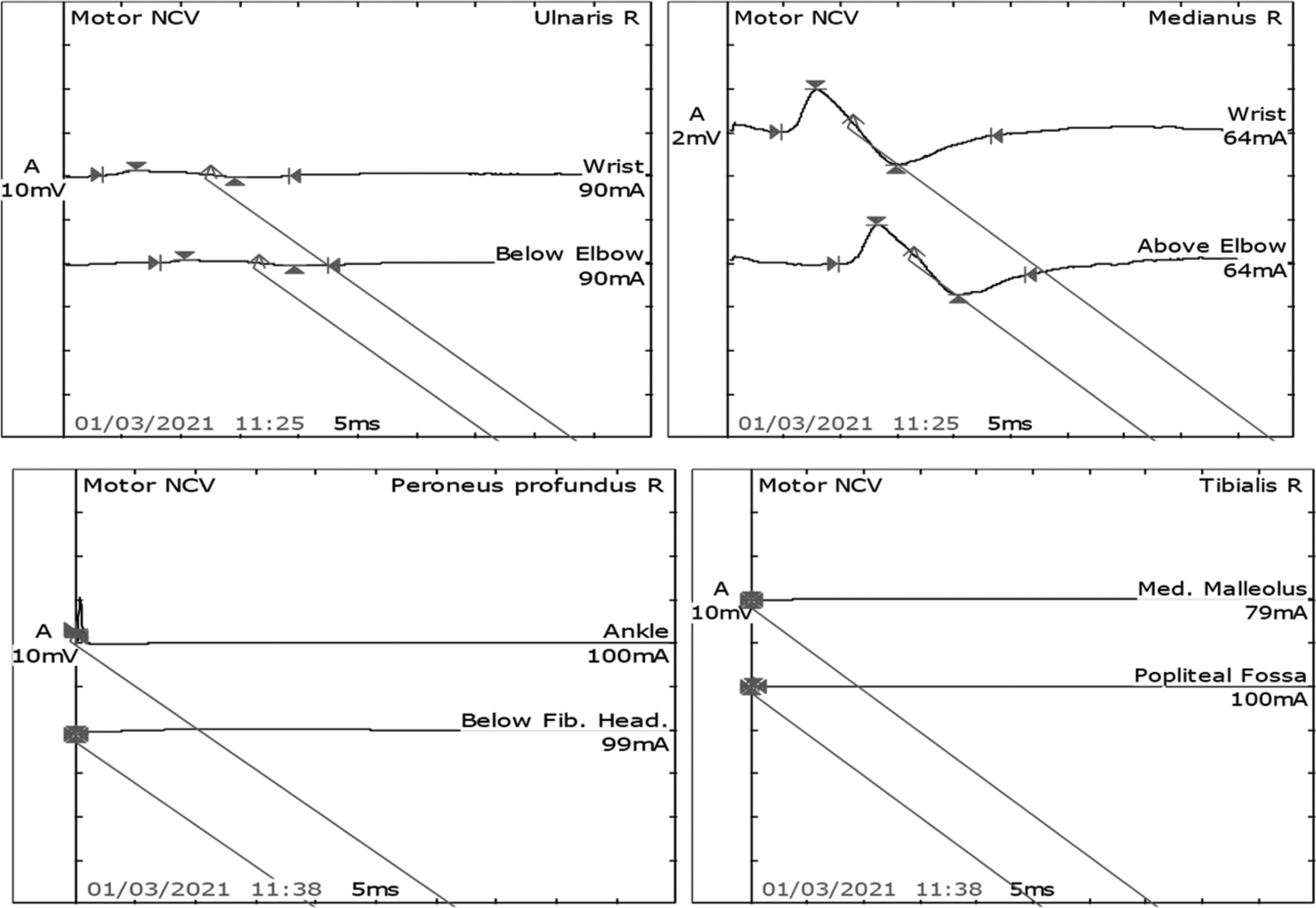
- Motor Nerve conduction study in the right median and ulnar nerves showed normal latency, reduced amplitude, and normal velocity. However, the right peronial and tibial nerves latency, amplitude, and velocity were not recordable.
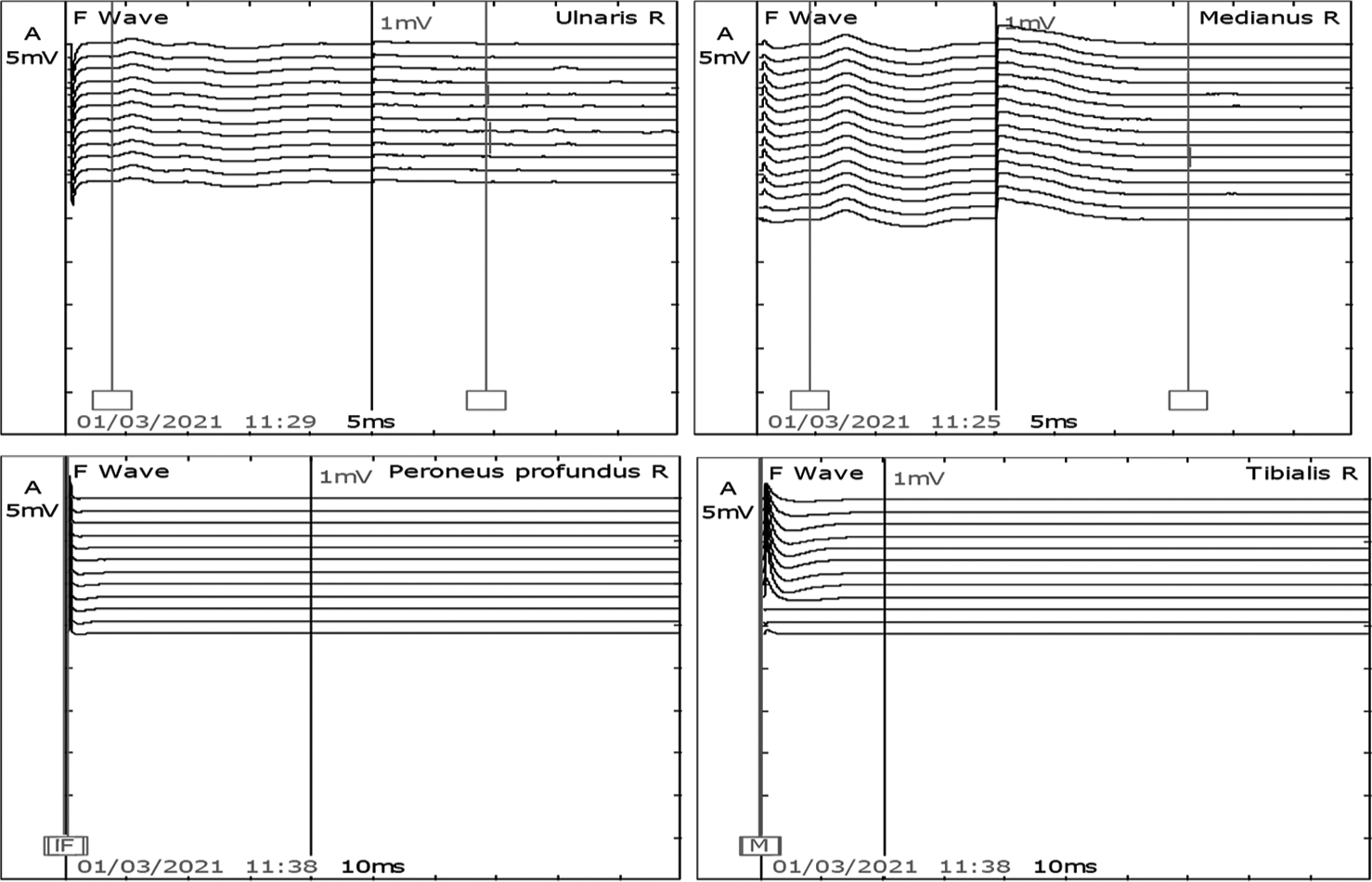
- F waves in the right median and ulnar nerves were present. However, peroneal and tibial nerve was not recordable.
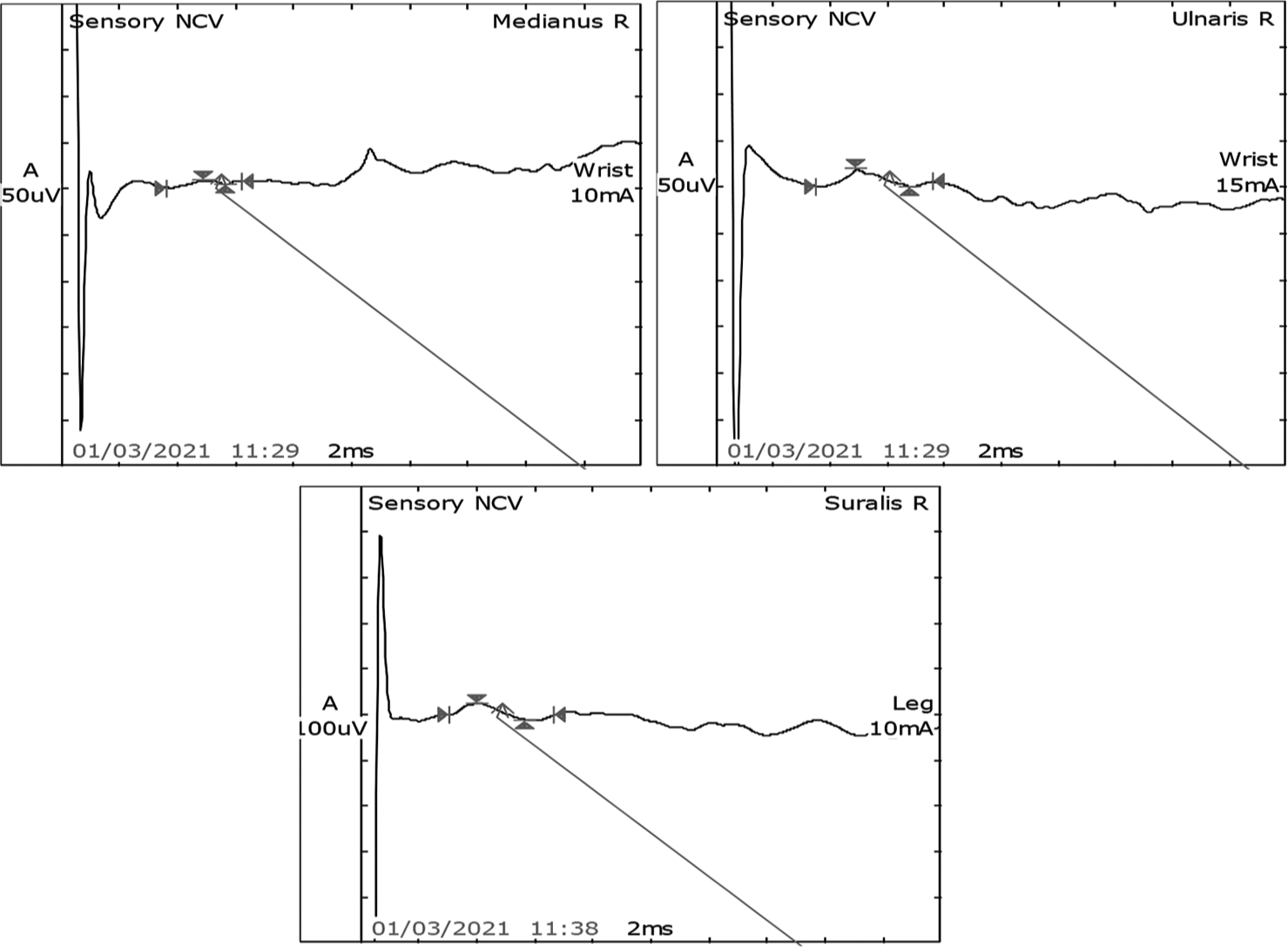
- Sensory nerve conduction study showed normal latency, amplitude, and velocity in the right median, ulnar, and sural nerves.
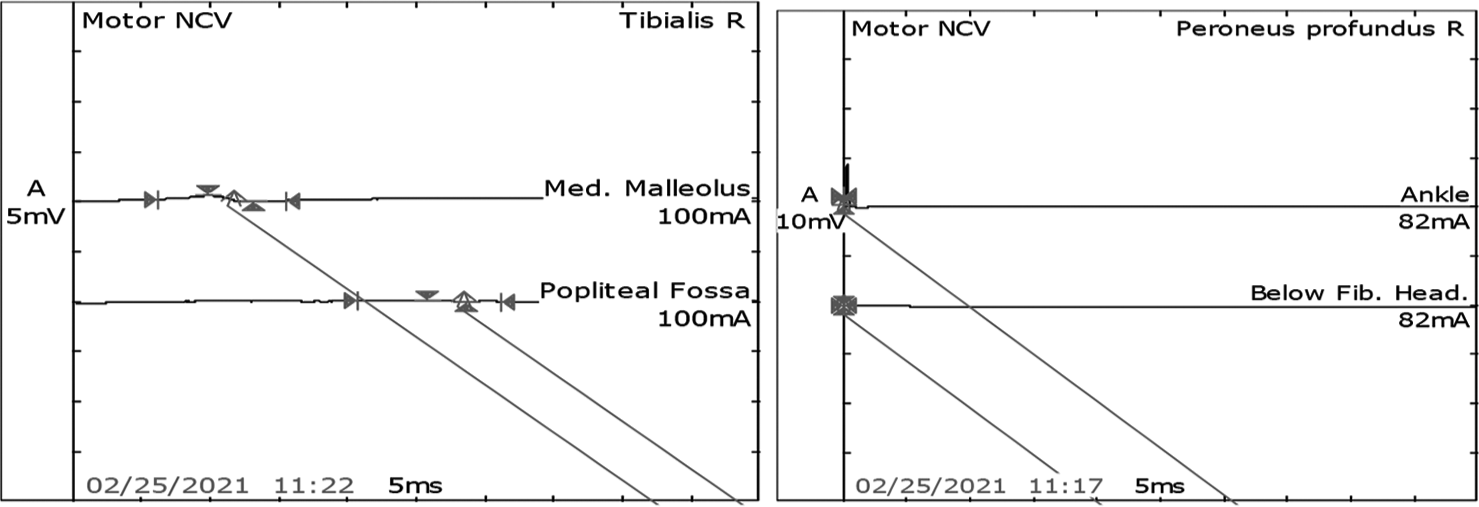
- Post-intravenous immunoglobulin motor nerve conduction study in the right tibial nerve showed mild improvement in increased latency, reduced amplitude, and reduced velocity. However, the right peronial was still not recordale.
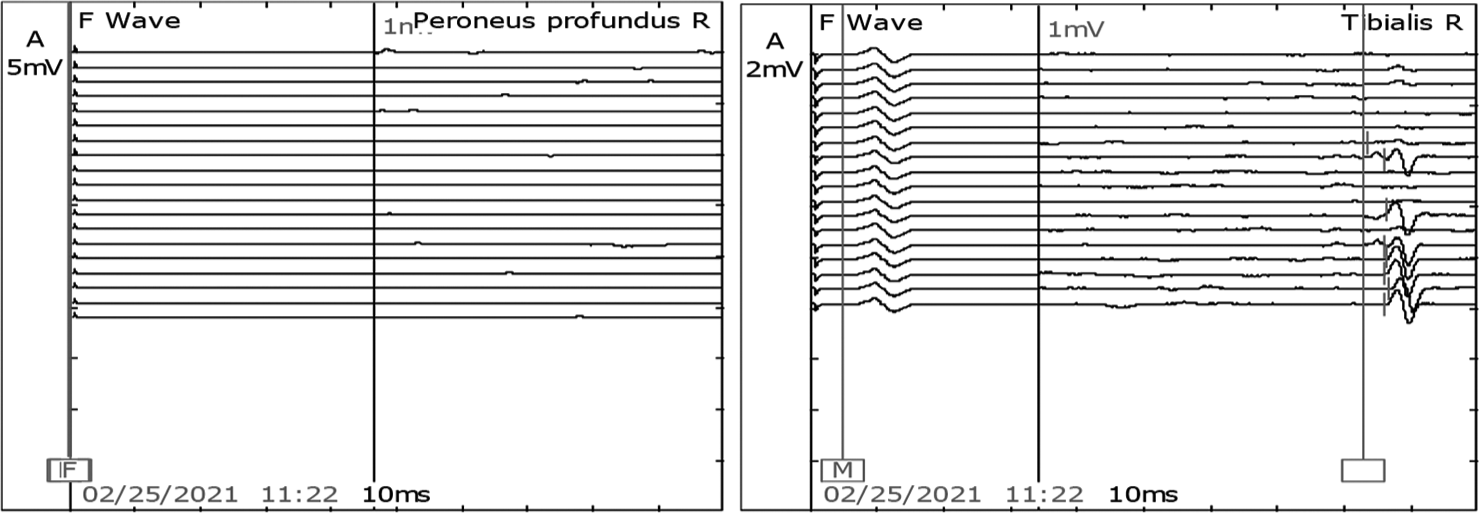
- Post-intravenous immunoglobulin F waves in the right tibial nerve was present. However, the right peroneal was not recordable.
DISCUSSION
Leptospirosis is a zoonosis, more common in tropical and subtropical regions. It can lead to mild or fulminant life-threatening illness and multiorgan failure. The incubation period is variable and ranges from 1 to 30 days.[1] Clinical diagnosis is difficult because leptospirosis presents with non-specific symptoms that overlap with another form of illness.[2] Our patient presented with hypotension, AKI and respiratory distress that has high case fatality ranging up to 50%. However, there was no thrombocytopenia, bleeding manifestations, or pulmonary hemorrhage, which is usually fatal.
Kidneys are the most common organ affected.[3] The case under review presented with AKI and had hyponatremia and hypokalemia at presentation, which responded to CRRT and showed complete renal recovery. Liver involvement is seen in 40% of cases. Our patient, too, had mildly deranged liver enzyme levels but remained anicteric with bilirubin levels <2 g/dL. Cardiac involvement is rare and seen in severe cases such as circulatory collapse, congestive cardiac failure, and non-specific ST-T changes.[4] Hypotension and moderate left ventricular dysfunction were seen at initial echocardiography. However, that also improved. On repeat, echocardiography showed an ejection fraction of 50%.
Central nervous system (CNS) involvement is seen in a severe form of leptospirosis. Leptospiral meningitis may present with altered mental status, which might be difficult to diagnose, especially when the patients are anicteric, as seen in our case.[1] The most common CNS complication is aseptic meningitis in about 25% of cases.[3] CSF analysis typically shows pleocytosis with mildly elevated protein levels, predominant polymorphonuclear cells initially, that are later replaced by lymphocytes. Other rare neurological complications are GBS, hemiplegia, and transverse myelitis.[1] Neurological complications might be seen months after the acute illness.[2] Our patient developed aseptic meningitis during the acute illness, and GBS developed after 7 days while the other systemic complications were improving. Critical care neuropathy was ruled out. He did not not have any sensory neuropathy. Within five days he was weaned off from mechanical ventilation support. Weakness in limbs developed after 7 days of extubation and removal of ventilatory support. On follow-up, the quadriparesis improved with mild residual weakness of the left proximal lower limb.
Commonly used tests for presumptive diagnosis of leptospirosis are the microscopic agglutination test (MAT) and IgM-enzyme linked immunosorbent assay (ELISA). MAT uses paired and convalescent serum, while IgMELISA uses a broadly reactive antigen and is useful for diagnosis during the acute phase of infection.[5] Doxycycline and intravenous Penicillin G are the drugs of choice. Third-generation cephalosporins are used in patients allergic to penicillin.[1] We have used tigecycline and ceftriaxone, and he responded. His weakness also responded to immunoglobulins, and he became ambulant without support.
CONCLUSION
Leptospirosis is a potentially serious illness and results in multi-organ failure requiring intensive care unit admission. Initial signs and symptoms and routine laboratory investigations can be nonspecific. Neurological complications are rare and might manifest late; hence, a follow-up of cases is essential. A high degree of suspicion and a detailed history of the risk factors and exposure are of most importance, especially in tropical countries.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Harrison’s principle of internal medicine (20th ed). New York: McGraw Hill; 2018. p. :1290-4.
- [Google Scholar]
- Available from: https://www.who.int/csr/don/en/who_cds_csr_eph_2002.23 [Last accessed on 2024 May 28]
- Available from: http://www.ncdc.gov.in.2015.national-guideline-diagnosis-case-management-prevention-and-control-ofleptospirosis [Last accessed on 2024 May 28]
- Leptospirosis in Coastal South India: A facility based study. Biomed Res Int. 2018;2018:1759125.
- [CrossRef] [Google Scholar]
- Leptospirosis diagnosis: Competancy of various laboratory tests. J Clin Diagn Res. 2014;8:199-202.
- [CrossRef] [Google Scholar]







