Translate this page into:
A rare presentation of rhabdomyolysis-associated acute kidney injury and hepatocellular dysfunction with persistent proximal weakness of lower limb
*Corresponding author: Adarsh Kumar, Department of Nephrology, VMMC and Safdarjung Hospital, New Delhi, India. adarshnephro081@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kumar A, Binoy R. A rare presentation of rhabdomyolysis-associated acute kidney injury and hepatocellular dysfunction with persistent proximal weakness of lower limb. Indian J Med Sci. doi: 10.25259/IJMS_237_2024
Abstract
Exercise-induced rhabdomyolysis (ERM) is a pathophysiological condition where skeletal muscles break down, releasing their contents into the bloodstream. Common complications include acute kidney injury (AKI), compartment syndrome, and disseminated intravascular coagulation, with AKI being the most serious. Rarely, ERM may also be associated with hepatocellular dysfunction, leading to confusion in diagnosis. The reason for elevated liver enzymes in this condition is not yet fully understood. In addition, persistent weakness in the lower limbs after rhabdomyolysis is rarely reported. In this particular case, the patient presented with these symptoms for the first time, prompting consideration of inherited metabolic disorders and genetic mutations. This case study highlights a rare instance of hepatocellular dysfunction and persistent limb weakness resulting from ERM, which necessitated prolonged dialysis for renal recovery.
Keywords
Rhabdomyolysis
Acute kidney injury
Hepatocellular dysfunction
Proximal lower limb weakness
INTRODUCTION
The severity of rhabdomyolysis presentation varies from asymptomatic creatine phosphokinase (CPK) elevations to life-threatening renal failure, compartment syndrome, and disseminated intravascular coagulation.[1-3] Its etiology includes marked exertion in untrained individuals, metabolic myopathies, and hyperthermia. There are very few reports of rhabdomyolysis associated with hepatocellular dysfunction. The mechanisms for hepatocellular dysfunction have not yet been elucidated; it may be attributed to liver damage caused by proteases released from injured muscle. In our patient, rare findings included severe transaminitis and prolonged weakness in the lower limbs, which prompted a detailed investigation to rule out all potential causes of hepatorenal involvement. It is believed that the reversible hepatocellular dysfunction in our patient was related to rhabdomyolysis. In addition, prolonged lower limb weakness following the recovery from acute kidney injury (AKI) has also been rarely reported. These findings have led us to present this case.
CASE REPORT
A 24-year-old male with no significant past medical or family history presented with complaints of fatigue, muscle pains, and decreased urine output 1 day after engaging in heavy exercise during physical training. His vital signs were as follows: blood pressure 126/80 mmHg, temperature 36.8°C, pulse 123/min, and respiration rate 18/min. Physical examination revealed tenderness to palpation of the lower extremities, especially over the thighs. Further investigation showed deranged kidney function tests (Urea/SCr: 156/8.0 mg/dL) and deranged liver function tests (Aspartate transaminase [AST]/alanine transaminase [ALT]: 5600/4880 U/L, prothrombin time-international normalized ratio – 1.6, and total bilirubin – 4.0 mg/dL). Due to hepatorenal involvement, a detailed microbiological work-up was conducted to rule out infectious causes. His blood and urine cultures were sterile; no malarial parasite was seen on the peripheral blood smear, and enzyme-linked immunosorbent assay for detection of antibodies to leptospira, scrub typhus, and dengue were negative. Hepatitis A, B, C, and E virus serology was also negative. His CPK level was 66,000 U/L, and his urine tested positive for myoglobin. He received alternate-day hemodialysis, but there was no improvement in urine output until the 14th day of admission. In the 2nd week, FibroScan, splenic-portal axis ultrasound (USG), and upper gastrointestinal endoscopy were conducted and found to be normal. A renal biopsy was performed, which showed severe acute tubular necrosis (ATN) with myoglobin cast. He exhibited improvement in renal and liver parameters later, and hemodialysis support was discontinued on the 26th day [Figure 1]. He was discharged on the 35th day but experienced proximal weakness in the lower limbs, for which a neurologist evaluated him. After normal nerve conduction velocity (NCV) and electromyography (EMG) tests, he was advised to undergo muscle biopsy and genetic testing if the weakness persisted for a prolonged period. He was discharged with instructions to follow-up in the outpatient department.
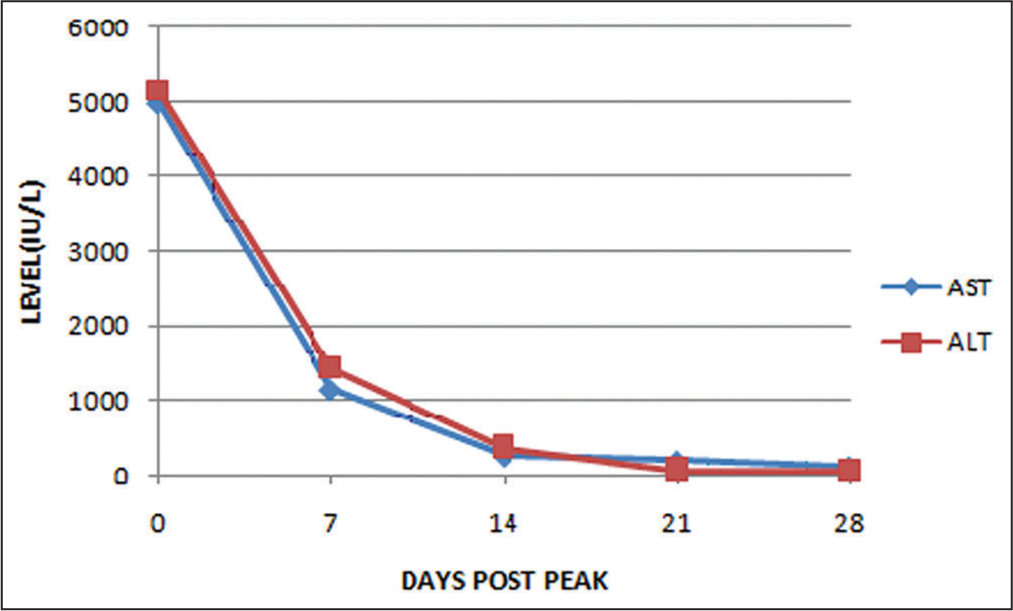
- The peak levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were observed on the second day of hospitalization, and it took approximately 28 days for these levels to return to normal. The serum levels of AST and ALT decreased in parallel with the pre-dialysis serum creatinine and creatine phosphokinase levels, indicating an association with rhabdomyolysis.
DISCUSSION
Rhabdomyolysis syndrome is characterized by the release of large quantities of potassium, aldolase, phosphate, myoglobin, CPK, lactate dehydrogenase, and AST into the circulation. When more than 100 g of muscle tissue is degraded, the plasma’s myoglobin binding capacity is overwhelmed, and free myoglobin causes renal morbidity through several mechanisms.[4,5] ALT is a cytosolic enzyme that is a specific marker of hepatic injury, while AST is present in several organs, including the liver, heart, kidneys, pancreas, and other organs. Few studies have demonstrated biochemical evidence of liver injury as a component of the systemic manifestation of massive rhabdomyolysis.[6-9] Akmal and Massry[6] reported that reversible hepatocellular dysfunction appears to occur in 25% of patients with rhabdomyolysis. Karcher et al.[7] reported the case of an obese patient who developed rhabdomyolysis with AKI and hepatocellular dysfunction. Kim et al.[8] and Park et al.[9] also observed a marked elevation of AST and ALT. The exact mechanism of hepatocellular damage in this syndrome is not well understood. Horl et al.[10] have demonstrated that proteolytic activity increases in patients with acute renal failure and rhabdomyolysis. Hepatocellular dysfunction in rhabdomyolysis may be attributed to liver damage caused by proteases released from injured muscle.
Hepatocellular dysfunction secondary to rhabdomyolysis is rare but should be considered when other causes are excluded.[11] Some studies have suggested a multifactorial hypothesis that involves a combination of hyperpyrexia, hypotension, and proteases released from injured muscles-induced liver injury.[8,9] Prothrombin time, bilirubin levels, and albumin levels are used to differentiate whether AST and ALT elevation is attributed to muscle injury solely or concomitant liver damage. The kidney is potentially affected in severe forms of rhabdomyolysis, where volume depletion, tubular obstruction due to heme pigment casts, and tubular injury from free iron all manifest as AKI.[12,13]
Our case is noteworthy for several reasons. First, it was characterized by severe transaminitis, which complicated the differential diagnosis by making it difficult to rule out other infectious or non-infectious causes of hepato-renal involvement common in tropical regions. Severe transaminitis is typically associated with viral hepatitis, drug-induced liver injury, or shock. In this case, we were able to exclude these causes due to the patient’s lack of drug use or toxin exposure, the absence of profound shock at the time of presentation, and negative viral hepatitis serology.
In addition, we ruled out other infectious causes of hepatorenal dysfunction, such as malaria, leptospirosis, dengue, typhoid, and bacterial sepsis, based on clinical and laboratory findings. The possibilities of hepatorenal syndrome or acute decompensation of underlying liver cirrhosis[14] were also ruled out after conducting a FibroScan, USG of the splenic-portal axis, and upper gastrointestinal endoscopy. Furthermore, no work-up for autoimmune or metabolic diseases was necessary, as the patient’s hepatocellular dysfunction improved. We believe that the hepatocellular dysfunction, indicated by hyperbilirubinemia, prolonged prothrombin time, and elevated transaminases, was likely secondary to rhabdomyolysis.[15] This conclusion is supported by the observation that both ALT and AST levels decreased in parallel with serum creatinine and CPK levels, suggesting an association with rhabdomyolysis.
Second, it required 14 sessions of hemodialysis over almost 1 month to aid in the recovery of renal function. After 4 weeks, urine output improved, and it took a further 10 days for the serum creatinine levels to decrease (reaching 1.8 mg% at the time of discharge). A renal biopsy was performed 3 weeks after admission as the patient remained dependent on dialysis for an extended period. The biopsy revealed severe ATN with a myoglobin cast.
Third, the extremely high CPK levels indicate significant muscle necrosis, suggesting that the patient may have suffered from compartment syndrome, characterized by painful swelling in the thigh that becomes more prominent during the 1st week of admission. The CPK level showed a decreasing trend during the illness but remained in the thousands until 1 month after the illness. Serum CPK levels begin to rise approximately 2–12 h after the onset of muscle injury, peak within 24–72 h, and then decline at a relatively constant rate of 39% of the previous day’s value. Serum creatinine is elevated to a greater extent than blood urea nitrogen, narrowing the normal 10:1 ratio of blood urea nitrogen to creatinine to a ratio of 6:1 or less. We observed a similar trend in the serum creatinine level in our case. Both AKI and increased release of creatinine from skeletal muscle contribute to the elevated serum concentration of creatinine.
Fourth, the patient experienced severe weakness in both lower limbs, to the point of being unable to walk. This led us to consider genetic causes of rhabdomyolysis, despite no prior history of similar episodes. NCV and EMG tests showed normal results. Further investigations, such as muscle biopsy and genetic analysis, were planned in case the patient’s lower limb weakness would not improve after a period of observation. The prolonged weakness in the upper part of both lower limbs could be due to extensive muscle necrosis, indicated by persistently high levels of CPK. A consistently elevated CPK level suggests ongoing muscle injury or the development of compartment syndrome.[16]
CONCLUSION
Severe hepatocellular dysfunction and prolonged proximal weakness of the lower limbs may be associated with exercise-induced rhabdomyolysis due to massive muscle necrosis.
Acknowledgment
The author is grateful for the institutional support that helped him in carrying out all possible investigations and advance management of this case.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Rhabdomyolysis and acute renal failure in a polymyositis patient. Mod Rheumatol. 2004;14:422-3.
- [CrossRef] [PubMed] [Google Scholar]
- Rhabdomyolysis: An evaluation of 475 hospitalized patients. Medicine (Baltimore). 2005;84:377-85.
- [CrossRef] [PubMed] [Google Scholar]
- Unmasking the Janus face of myoglobin in health and disease. J Exp Biol. 2010;213:2734-40.
- [CrossRef] [PubMed] [Google Scholar]
- Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62-72.
- [CrossRef] [PubMed] [Google Scholar]
- Reversible hepatic dysfunction associated with rhabdomyolysis. Am J Nephrol. 1990;1:49-52.
- [CrossRef] [PubMed] [Google Scholar]
- Rhabdomyolysis in an obese patient after total knee arthroplasty. Br J Anaesth. 2006;97:822-4.
- [CrossRef] [PubMed] [Google Scholar]
- Rhabdomyolysis after laparoscopic radical nephrectomy. Korean J Anesthesiol. 2010;59:41-4.
- [CrossRef] [PubMed] [Google Scholar]
- Hyperkalemia in a patient with rhabdomyolysis and compartment syndrome. Korean J Anesthesiol. 2010;59:37-40.
- [CrossRef] [PubMed] [Google Scholar]
- Role of proteases in hypercatabolic patients with renal failure. Kidney Int Suppl. 1983;24:37-42.
- [Google Scholar]
- Massive rhabdomylosis; a rare cause of hepatocellular dysfunction. Am J Case Rep. 2018;29:105-8.
- [CrossRef] [PubMed] [Google Scholar]
- Rhabdomyolysis and acute renal failure. Crit Care Nurse. 1990;10:32-6.
- [CrossRef] [PubMed] [Google Scholar]
- Management of decompensated cirrhosis. Clin Med (Lond). 2018;18:S60-5.
- [CrossRef] [PubMed] [Google Scholar]
- Liver aminotransferases are elevated with rhabdomyolysis in the absence of significant liver injury. J Med Toxicol. 2010;6:294-300.
- [CrossRef] [PubMed] [Google Scholar]
- A case of occult compartment syndrome and nonresolving rhabdomyolysis. J Gen Intern Med. 2008;23:871-4.
- [CrossRef] [PubMed] [Google Scholar]






