Translate this page into:
Prognostic significance of programmed death ligand 2 expression in tumor-infiltrating lymphocytes of triple-negative breast cancer
*Corresponding author: Jabed Iqbal, Department of Anatomical Pathology, Singapore General Hospital, Singapore. jabed.iqbal@singhealth.com.sg
-
Received: ,
Accepted: ,
How to cite this article: Ong C, Jegannathan N, Iqbal J. Prognostic significance of programmed death ligand 2 expression in tumor-infiltrating lymphocytes of triple-negative breast cancer. Indian J Med Sci. doi: 10.25259/IJMS_141_2024
Abstract
Objectives:
Triple-negative breast cancer (TNBC) is a highly aggressive subtype of breast cancer characterized by the absence of estrogen receptor (ER), progesterone receptor (PR), and overexpression of human epidermal growth factor receptor 2 protein. Patients with high expression of programmed death ligand 1 (PD-L1) and programmed cell death protein 1 (PD-1) have been found to have better prognosis and increased response to anti-PD-1/PDL1 immunotherapy. However, the role programmed death ligand 2 (PD-L2), the other known ligand of PD-1, plays in PD-1/PD-L1 checkpoint pathway has not been well studied. Therefore, this project aims to investigate (1) the relationship between PD-L2 expression in tumor infiltrating lymphocytes (TILs) and patient clinicopathological features, (2) whether PD-L2 can serve as a predictor of patient survival, and (3) the association of PD-L2 expression with the infiltration of relevant immune cell types in the tumor microenvironment.
Materials and Methods:
Two hundred and ninety-six (296) TNBC cases diagnosed between 2003 and 2013 in Singapore General Hospital were used in this study to create tissue microarray for immunohistochemistry with several antibodies.
Results:
Patients with PD-L2 expression were found to have significantly improved disease-free survival (hazard ratio [HR] 0.51; P = 0.0362) and overall survival (HR 0.43; P = 0.0379) compared to patients who have negative PD-L2 expression. PD-L2+ TILs correlate significantly with CD3+ T-cells (P = 0.00776) and CD20+ B-cells (P = 0.001019) infiltration in the stromal compartments and intratumoral CD38+ plasma cells (P = 0.048869) infiltration.
Conclusion:
Like PD-L1, PD-L2 positivity in TILs was found in our study to indicate a better prognosis compared to PD-L2-negative patients.
Keywords
PD-1
Programmed death ligand 1
Triple-negative breast cancer
Immunotherapy
INTRODUCTION
Triple-negative breast cancer (TNBC) is a heterogeneous subtype of breast cancer characterized by the absence of estrogen receptor (ER), progesterone receptor (PR), and overexpression of human epidermal growth factor receptor 2 (HER2) protein. TNBC is biologically aggressive but limited in therapeutic options and response rates are often poor. Lacking a target receptor, TNBC is typically managed by systemic cytotoxic chemotherapy, often leading to relapse.[1,2]
TNBC exhibits a high level of tumor infiltrating lymphocytes (TILs) and indicates an encouraging outlook of utilizing immunotherapy as a new form of treatment. In 2019, Food and Drug Administration approved atezolizumab, a monoclonal antibody targeting programmed death ligand-1 (PD-L1) in combination with chemotherapy drug nabpaclitaxel for the treatment of patients with PD-L1-positive unresectable, locally advanced, or metastatic TNBC.[3]
Programmed cell death protein 1 (PD-1) or (CD279) protein is one of the most important and well-known immune response pathway.[4] PD-1 has two ligands: PD-L1 and programmed death ligand 2 (PD-L2). Like PD-L1, PD-L2 is also expressed on TILs, tumor cells, and non-immune cells. Both PD-L1 and PD-L2 expressions can be induced by inflammatory cytokines and oncogenic signaling due to mutations.[5,6] PD-L2 expression is generally induced by Th2-associated cytokines.
The role PD-L2 plays in PD-1/PD-L1 checkpoint pathway has not been well studied. Studies have reported that PD-L2 acts similarly to PD-L1 in inhibiting cytokine production, T-cell proliferation, and cytolysis.[7-10]
Current screening of patients eligible for treatment with PD-1/PD-L1 checkpoint inhibitors is based primarily on the expression of PD-L1 in tumor cells at baseline (positive vs. negative). Given the importance role PD-L2 may play in the PD-1 immune interactions within the tumor microenvironment, understanding the relative contribution of PD-L2 will help to devise better prognostic indicators for breast cancer patients who will benefit from the therapy and maximize the effectiveness of immunotherapeutic treatments. This project aims to examine the differential expression of PD-L2 in TILs in relation to patient clinicopathological features, the association of PD-L2 expression with the infiltration of other relevant immune cell types in the TNBC tumor microenvironment and whether PD-L2 can serve as a predictor of patient survival.
MATERIALS AND METHODS
Patients and tumors
Archival formalin-fixed, paraffin-embedded biopsy samples (n = 296) taken at the time of surgery of patients diagnosed with TNBC from 2003 to 2013 at the Department of Anatomical Pathology, Division of Pathology, Singapore General Hospital were analyzed. Clinicopathological features including patient age, ethnicity, tumor size, grade and subtype, lymphovascular invasion, and lymph node stage were examined [Table 1].
| Factors | PD-L2 | ||
|---|---|---|---|
| Negative | Positive | P-value | |
| Age (years) | 55.3 | 52.1 | 0.2642 |
| Tumor size (mm) | |||
| ≤20 | 85 | 5 | 0.9254 |
| >20 | 200 | 15 | |
| Tumor grade | |||
| ½ | 46 | 3 | 0.8932 |
| 3 | 239 | 17 | |
| Lymph node stage (pN) | |||
| 0 | 118 | 7 | 0.5212 |
| 1 | 45 | 5 | |
| 2 | 27 | 2 | |
| 3 | 15 | 0 | |
| Lymphovascular invasion | |||
| Absent | 186 | 13 | 0.9474 |
| Present | 99 | 7 | |
| Ethnicity | |||
| Chinese | 231 | 15 | 0.6366 |
| Indian | 13 | 1 | |
| Malay | 10 | 3 | |
| Other | 31 | 7 | |
TNBC: Triple-negative breast cancer, PD-L2: Programmed death ligand 2
Immunohistochemistry (IHC) analysis
Tissue microarray sections of 4 µm thickness were stained with anti-PD-L2 antibody (D7UHC), antibodies against CD3, CD4, CD8, FOXP3, CD20, CD38, CD44, CD138, CD163, CXCR4, E-cadherin, PD-1, PD-L1, as well as ER, PR and HER2 [Table 2]. PD-L2 and PD-L1 staining was scored based on expression in TILs, defined as ≥1 TILs count. TILs expressing CD3, CD4, CD8, FOXP3, CD20, CD38, CD44, CD138, and CD163 staining were evaluated separately in the intra-tumoral region and stromal region. Intra-tumoral TILs were defined as immune cells that are in contact with each other and cancer cells but are not the with the tumor stroma.[11] Stromal TILs were defined as immune cells that exist within the tumor stroma but are not in contact with cancer cells.[11] TILs with respective markers that occupy the intra-tumoral or stromal area were quantified in percentages and scored manually by two observers. E-cadherin and CXCR4 expressions were also examined in both the membrane and cytoplasm of tumor cells.
| Antibody | Clone | Dilution | Source | Labeling pattern |
|---|---|---|---|---|
| ER | SP1 | 1:50 | Thermo Scientific Lab Vision RM 9101-S | Nuclear |
| PR | SP2 | 1:200 | Thermo Scientific Lab VisionRM9102-S | Nuclear |
| HER2 | SP3 | 1:200 | Thermo Scientific Lab VisionRM9103-S | Membranous |
| PD-L2 | D7U8C | 1:50 | CST #82723 | Membrane |
| CD3 | Ra | 1:200 | Dako A0452 | Membrane |
| CD4 | 4B12 | 1:100 | Leica NCL-L-CD4-368 | Membrane |
| CD8 | 4B11 | 1:100 | Leica NCL-L-CD8-4B11 | Membrane |
| CD38 | SPC32 | 1:50 | NCL-CD38-290 | Membranous |
| CD20 | L26 | 1:200 | Dako M0755 | Membranous and/or cytoplasmic |
| FOXP3 | 236A/E7 | 1:200 | Abcam ab20034 | Nuclear |
| CD44 | DF1485 | 1:50 | Dako M7082 | Membrane |
| CD138 | MI15 | 1:400 | Dako M7228 | Membrane |
| CD163 | 10D6 | 1:200 | Cell Marque 163M-16 | Membrane |
| CXCR4 | UMB2 | 1:100 | Abcam ab12484 | Membrane |
| E-cadherin | MCH-38 | 1:30 | Dako M3612 | Membrane/cyto |
| PD-1 | NAT105 | 1:200 | Cell Marque 315M-96 | Cyto |
| PD-L1 | SP142 | RTU | Roche 741–4860 | Membrane |
| SP263 | RTU | Roche 741–4905 | Membrane/cyto | |
| 22C3 | RTU | Dako SK006 | Membrane | |
| E1L3N | 1:600 | CST 13684S | Membrane/cyto |
IHC: Immunohistochemistry, TNBC: Triple-negative breast cancer, FFPE: Formalin-fixed, paraffin-embedded, ER: Estrogen receptor, PR: Progesterone receptor, PD-L: Programmed death ligand, HER2: human epidermal growth factor receptor 2, FOXP3: Forkhead box P3, RTU: Ready to use
Statistical analysis
Follow-up data were extracted from medical records. Disease-free survival (DFS) was defined as the time from diagnosis to relapse. Overall survival (OS) was defined as from diagnosis to death/date of last follow-up. Statistical analysis was performed using Microsoft Excel 2010. Kaplan-– Meier analysis was performed to estimate survival outcomes. Clinicopathological parameters and PD-L1 status were adjusted in multivariate Cox regression to examine the effect of PD-L2 status on patient survival by another researcher.
RESULTS
Association of PD-L2 expression with clinicopathological characteristics in TNBC patients
Membrane PD-L2 expression in TILs was seen in 22.5% (63/280) of TNBC cases. A few patients were excluded in the analysis due to loss of tissue during immunoassay. The number does not match because not all clinicopathological information was available for all patients. IHC staining of PD-L2 in TILs is shown in Figure 1. Table 1 summarizes the univariate analysis of clinicopathological data association with PD-L2 protein expression. None of the clinicopathological parameters was found to be significantly associated with PDL2 protein expression.

- IHC staining of programmed death ligand 2 expression in tumor infiltrating lymphocytes. IHC: Immunochemistry. (a) ×5 and (b) ×40 magnification.
Increased PD-L2 expression correlates with better survival in TNBC patients
Kaplan–Meier survival curves of TNBC patients correlating with PD-L2 expression are shown in Figure 2. TNBC patients with PD-L2 expression were found to have significantly improved DFS (hazard ratio [HR], 0.51; 95% confidence interval, 0.27– 0.96; P= 0.0362) [Figure 2a] and OS (HR, 0.43; 95% confidence interval, 0.20–0.95; P= 0.0379) [Figure 2b] compared to patients who have negative PD-L2 expression. Moreover, the association of PD-L2 expression with better survival outcomes in both DFS (HR, 0.48; 95% confidence interval, 0.24–0.97; P= 0.0403) and OS (HR, 0.44; 95% confidence interval, 0.19–1.03; P= 0.058) of TNBC patients remained significant in multivariate analysis after adjustment for PD-L1 expression [Table 3].
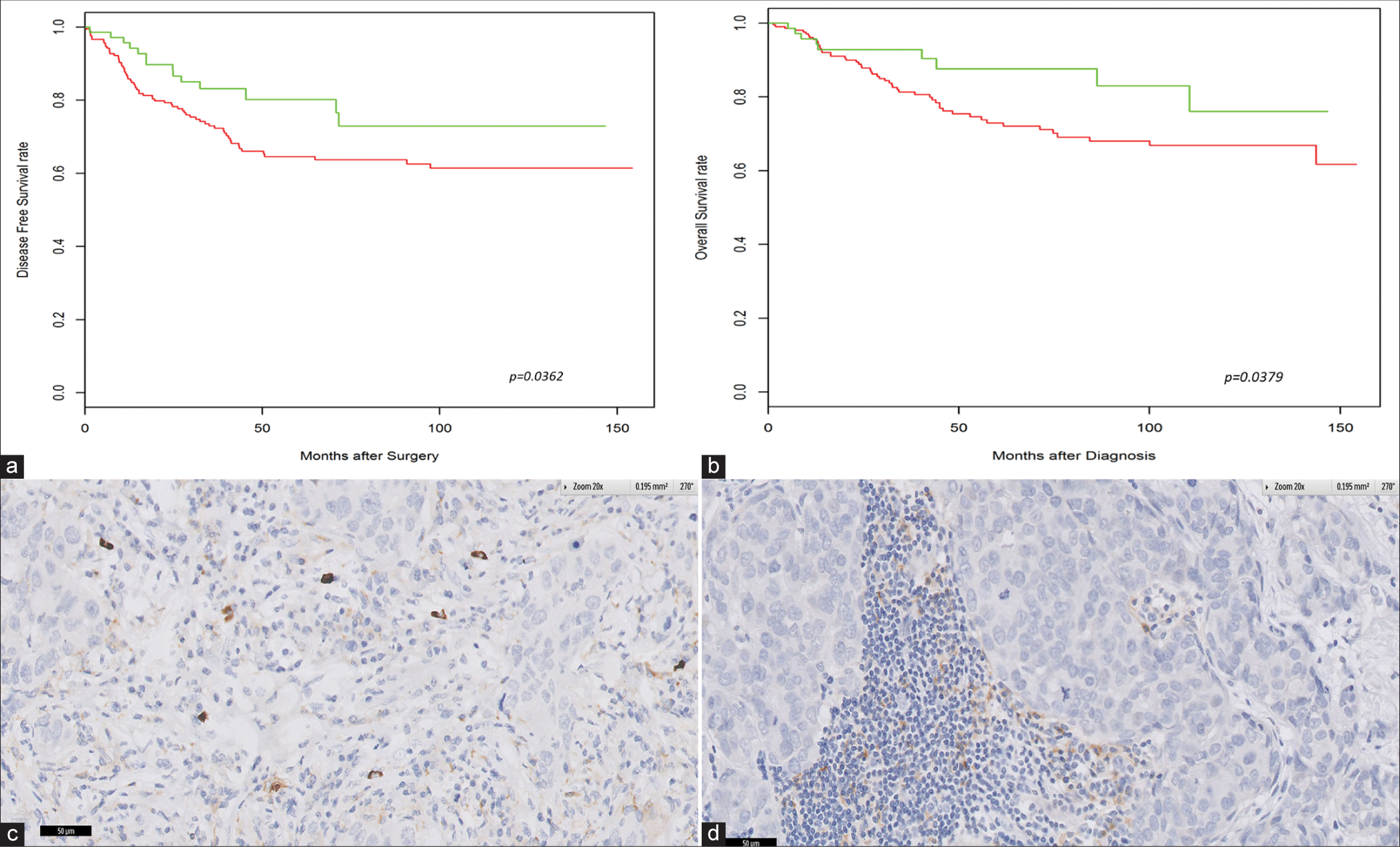
- High densities of programmed death ligand (PD-L2)-positive cells are associated with better clinical outcome in triple negative breast cancer. Kaplan–Meier analysis of (a) disease-free survival and (b) overall survival. (c and d) Correlation of PD-L2 positivity in tumor cells with PD-L1 positivity in the tumor cell membrane.
| No. of events | No. of patients | HR (95% CI) | P value | |
| Unadjusted cox regression of DFS | ||||
| PD-L2 (TILS) | ||||
| Negative | 71 | 218 | Reference | |
| Positive | 11 | 63 | 0.51 (0.27, 0.96) | 0.0362 |
| Adjusted by age, grade, size and lymph node stage | ||||
| PD-L2 (TILS) | ||||
| Negative | 62 | 171 | Reference | |
| Positive | 11 | 45 | 0.64 (0.33, 1.22) | 0.1716 |
| Adjusted by PD-L1 | ||||
| PD-L2 (TILS) | ||||
| Negative | 67 | 208 | Reference | |
| Positive | 9 | 58 | 0.48 (0.24, 0.97) | 0.0403 |
| No. of events | No. of patients | HR (95% CI) | P value | |
| Unadjusted cox regression of OS | ||||
| PD-L2 (TILS) | ||||
| Negative | 55 | 217 | Reference | |
| Positive | 7 | 63 | 0.43 (0.20, 0.95) | 0.0379 |
| Adjusted by age, grade, size and lymph node stage | ||||
| PD-L2 (TILS) | ||||
| Negative | 48 | 170 | Reference | |
| Positive | 7 | 45 | 0.53 (0.24, 1.17) | 0.1171 |
| Adjusted by PD-L1 | ||||
| PD-L2 (TILS) | ||||
| Negative | 52 | 208 | Reference | |
| Positive | 6 | 58 | 0.44 (0.19, 1.03) | 0.058 |
DFS: Disease-free survival, OS: Overall survival, PD-L: Programmed death ligand, TILS: Tumor infiltrating lymphocytes, CI: Confidence interval, HR: Hazard ratio
Correlation of PD-L2 expression in TILs and tumor cells with infiltrating immune cell populations
The density of PD-L2+ TILs correlates significantly with the densities of CD3+ T-cells (P = 0.00776) and CD20+ B-cells (P = 0.001019) in the stromal compartments [Figure 3a and b]. High density of PD-L2+ TILs was found to also correlate with densities of CD38+ plasma cells (P = 0.048869) in the intratumoral region [Figure 3c]. On the other hand, high density of PD-L2+ TILs was not found to be significantly associated with immune infiltration of FOXP3+ T regulatory cells, CD4+ and CD8+ T-cells, CD44+ effector-memory T-cells, CD138+ plasma cells, CD163+ macrophage/monocytes, co-expression of CXCR4, and E-cadherin in tumor cells. In addition, PD-L2+ TILs were not significantly associated with PD-1+ immune infiltrates or PD-L1+ TILs.
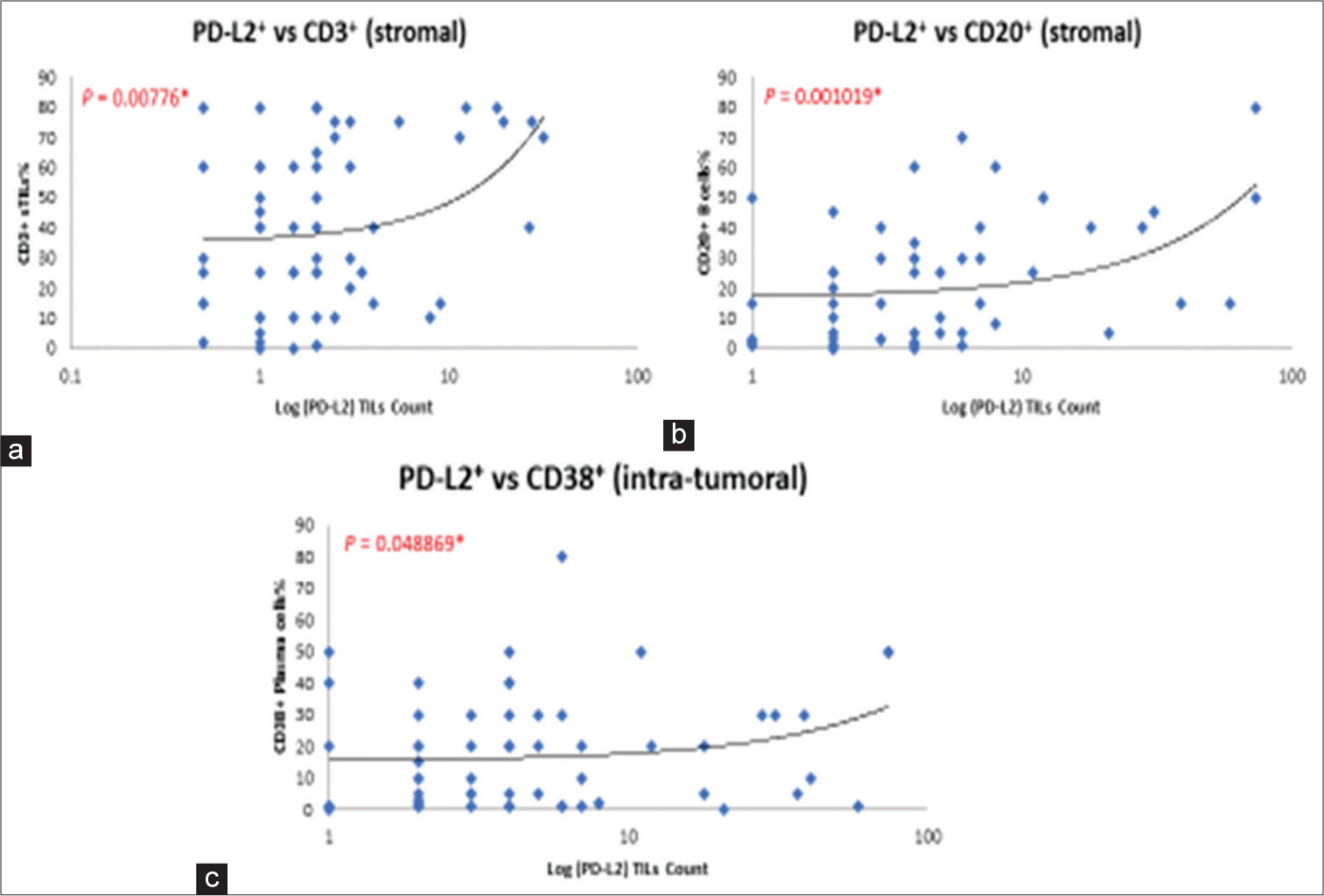
- (a and b) Correlation of density in programmed death ligand 2 (PD-L2)-positive tumor infiltrating lymphocytes (TILs) with CD3+ T-cells and CD20+ B-cells (stromal region). (c) Correlation of density in PD-L2-positive TILs with CD38+ plasma cells (intratumoral region).
Correlation of PD-L2 with PD-L1 expression tumor cells in the tumor microenvironment
PD-L2 positivity in tumor cells correlated significantly with PD-L1 positivity in the tumor cell membrane (P = 0.017221), as shown in Figure 2c and d.
DISCUSSION
In this study, we found that PD-L2 expression in TILs was significantly associated with better DFS and OS in TNBC patients. The association remained significant after correction for PD-L1 positivity indicating that PD-L2 function could be independent of PD-L1 expression and may serve as a potential prognostic marker for DFS and OS in TNBC patients. Furthermore, the presence of PD-L2-positive immune infiltrates was found to correlate with infiltration of various stromal immune cell populations in the TNBC microenvironment.
Compared to the extensively studied PD-1/PD-L1 pathway, there are limited studies that have looked into the role of PD-L2 as a prognostic indicator in cancer patients or as a potential biomarker for predicting clinical response to anti-PD-1/PD-L1 therapy. Studies have reported that high PD-L2 positivity correlated with worse survival in esophageal and colorectal cancer, but with a favorable survival outcome in melanoma and colorectal cancer in contrast.[12] Our study is the first in reporting the potential prognostic value of PD-L2 in TNBC with the largest cohort and association of PD-L2-positive TILs with infiltration of specific immune cell populations in the TNBC tumor microenvironment, as to the best of our knowledge.
PD-L2 positivity was similarly reported to be a significant predictor of progression-free survival in HNSCC patients.[13] The significance also remained after correction for PD-L1 status. However, contradictory findings were reported in another study that pooled 192 breast cancer cases of stage I, II, III.[14] In this study, PD-L2 expression was not found to be significantly associated with OS or DFS, while PD-L1 was found to be significantly associated with better OS and suggested to be a positive prognostic biomarker in breast cancer. The association of PD-L1-positive TILs with better survival outcomes was consistent with previous reports in TNBC and breast cancer in overall that indicated a strong antitumor immune response leading to PD-L1 expression.[15-17] However, this study observed younger age at diagnosis, lymph node positivity, and recurrence at distant sites in association with PD-L2 expression. The discordance between findings in overall breast cancer and our selected TNBC cases points to the differences in the role of PD-L2 among various breast subtypes, with an emphasis of a higher importance of PD-L2 plays in anti-tumor responses in specifically TNBC, as shown in our study.
The correlation of PD-L2 and PD-L1 in TNBC tumor cells indicated a possible common pathway of upregulation. The proximity of PD-L1 and PD-L2 genes in chromosome 9 could partly explain the observed correlation between these two proteins.[16] PDCD1LG2 (encoding PD-L2) expression was strongly associated with interferon gamma response gene signature, which is similar to the reported PD-L1-related cytokine expressions.[8,10,18] These studies provide supportive evidence that PD-L1 and PD-L2 may function similarly in immune regulation and evasion of antitumor immunity in tumor cells.
At present, there is neither approved anti-PD-L2 inhibitor nor any anti-PD-L2 inhibitor in clinical trials. A pre-clinical model that showed tumor resistance to anti-PD-L1 antibody alone in PD-L2 expressing tumors could be overcome by combined treatment of both anti-PD-L1 antibody and anti-PD-L2 antibody.[8] A recent study also demonstrated that by engineering a soluble decoy PD-1 molecule that can bind to both PD-L1 and PD-L2, the mutant resulted in T-cell activation and superior anti-tumor efficacy in highly PD-L2 expressing animal model of human ovarian cancer.[18]
As we found improved survival with PD-L2 expression in TNBC, we hypothesized that PD-L2 expression in TILs is correlated with an “immune-activated” tumor microenvironment with populations of infiltrating immune cells that are associated with improved patient survival. In our study, PD-L2 expressing TILs were found to correlate with densities of intra-tumoral CD38+ plasma cells, CD3+ T-cells, and CD20+ B-cells in the stromal compartments. Adding intra-tumoral CD38+ plasma cell density to the standard clinicopathological parameters was shown to increase prognostic value for both DFS and OS in our previous study.[19] This provides an indirect support for the potential use of PD-L2 expression as an additional prognostic marker in a combinatorial panel in TNBC patients.
Interestingly, we did not find any association of PD-L2 expression with densities of CD4+ and CD8+ T-cells. CXCR4 and E-cadherin protein or mRNA in tumor cells were not found to associate with PD-L2 expression in our cohort as well. This suggests that PD-L2 may not be involved in the process of epithelial-to-mesenchymal transition. Immune-related gene expressions of PD-L2 need to be analyzed to look for any particular clustering of gene signature for further comparison and validation of the interpretation of our data.
Limitations of the study include practical differences in antibodies, staining methods, interobserver errors in scoring, use of TMA cores rather than whole slide images, and some differences in sample sizes among sample groups due to loss of samples in antibody staining and handling.
CONCLUSION
We showed that PD-L2 expression in TILs is associated with better clinical outcomes independent of PD-L1 status. PD-L2 expression correlates with PD-L1 expression in tumor cells indicating a possible similar molecular pathway involved in oncogenic signaling of TNBC cells. PD-L2 positivity in TILs is linked with infiltration of subsets of immune cells that have been associated with better prognosis. However, the utility of including PD-L2 in selection criteria to identify patients likely to benefit from anti-PD-1/PD-L1 immunotherapy remains unclear. Lack of literature documenting PD-L2 pathways in TNBC and other cancers warrants an urgent need for further characterization of the PD-L2 immune pathway. Possibly, using combined PDL1 and PDL2 expression as a biomarker might help to select patients with higher chance of responding to immunotherapy.
Acknowledgments
This study was supported by the Singapore National Medical Research Council Transition Award (NMRC/TA/0041/2015) and the A*STAR Biomedical Research Council, National Medical Research Council Stratified Medicine Program Office (SMPO201302).
Ethical approval
This study was approved by the SingHealth Centralized Institutional Review Board (CIRB Ref: 2019/2818).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- An overview of triple-negative breast cancer. Arch Gynecol Obstet. 2016;293:247-69.
- [CrossRef] [PubMed] [Google Scholar]
- Triple-negative breast cancer: A review of conventional and advanced therapeutic strategies. Int J Environ Res Public Health. 2020;17:2078.
- [CrossRef] [PubMed] [Google Scholar]
- FDA approves atezolizumab for PD-L1positive unresectable locally advanced or metastatic triple-negative breast cancer. 2019. Available from: https://www.fda.gov [Last accessed on 2024 Jun 30]
- [Google Scholar]
- PD-L1 and PD-L2 expression correlated genes in non-small-cell lung cancer. Cancer Commun (Lond). 2019;39:30.
- [CrossRef] [PubMed] [Google Scholar]
- Combination therapy with PD-1 or PD-L1 inhibitors for cancer. Int J Clin Oncol. 2020;25:818-30.
- [CrossRef] [PubMed] [Google Scholar]
- Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108-21.
- [CrossRef] [PubMed] [Google Scholar]
- Immune suppression by PD-L2 against spontaneous and treatment-related antitumor immunity. Clin Cancer Res. 2019;25:4808-19.
- [CrossRef] [PubMed] [Google Scholar]
- Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression [published correction appears in Cell Rep 2019;29:3766] Cell Rep. 2017;19:1189-201.
- [CrossRef] [PubMed] [Google Scholar]
- PD-L2 is expressed on activated human T cells and regulates their function. Mol Immunol. 2011;48:2214-9.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation between PD-L2 expression and clinical outcome in solid cancer patients: A meta-analysis. Front Oncol. 2019;9:47.
- [CrossRef] [PubMed] [Google Scholar]
- The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259-71.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic relevance of programmed cell death 1 ligand 2 (PDCD1LG2/PD-L2) in patients with advanced stage colon carcinoma treated with chemotherapy. Sci Rep. 2020;10:22330.
- [CrossRef] [PubMed] [Google Scholar]
- PD-L2 based immune signature confers poor prognosis in HNSCC. Oncoimmunology. 2021;10:1947569.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol. 2016;47:78-84.
- [CrossRef] [PubMed] [Google Scholar]
- PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol. 2015;26:1488-93.
- [CrossRef] [PubMed] [Google Scholar]
- Triple-negative breast cancers with amplification of JAK2 at the 9p24 locus demonstrate JAK2-specific dependence. Sci Transl Med. 2016;8:334ra53. Erratum: Sci Transl Med 2019;11:eaaw6162
- [CrossRef] [PubMed] [Google Scholar]
- Programmed death ligand 2 in cancer-induced immune suppression. Clin Dev Immunol. 2012;2012:656340.
- [CrossRef] [PubMed] [Google Scholar]
- Neutralizing PD-L1 and PD-L2 enhances the efficacy of immune checkpoint inhibitors in ovarian cancer. bioRxiv 2020
- [CrossRef] [Google Scholar]
- High densities of tumor-associated plasma cells predict improved prognosis in triple negative breast cancer. Front Immunol. 2018;9:1209.
- [CrossRef] [PubMed] [Google Scholar]







