Translate this page into:
Adenovirus IgG and respiratory syncytial virus IgG seroprevalence in chronic obstructive pulmonary disease
*Corresponding author: Gulcin Alp Avcı, Department of Basic Medical Sciences, Gulhane Faculty of Dentistry, Health Sciences University, Ankara, Turkey. alp.gulcin@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Emniyet Sert A, Alp Avcı G, Arslan S. Adenovirus IgG and respiratory syncytial virus IgG seroprevalence in chronic obstructive pulmonary disease. Indian J Med Sci. 2024;76:61-6. doi: 10.25259/IJMS_207_2023
Abstract
Objectives:
Chronic obstructive pulmonary disease (COPD) is the inflammatory response of the airways against harmful gasses and particles in the lungs. It is an important cause of morbidity and mortality in all countries of the world due to progressive airway restriction. The acute exacerbation phase of COPD is usually triggered by bacterial or viral infections of the airway. The aim of this study was to determine the seroprevalence of respiratory syncytial virus (RSV)-immunoglobulin G (IgG) and adenovirus-IgG in COPD patients.
Materials and Methods:
Seroprevalence of RSV-IgG and adenovirus-IgG was investigated by enzyme-linked immunosorbent assay method in serum samples taken from 172 (107 male/65 female) patients being treated for COPD.
Results:
In the RSV-IgG study, 42.5% of the samples were positive, 49.4% were negative, and 8.1% gray-zone. In the adenovirus IgG study, 30.2% of the samples were positive, 61.6% negative, and 8.2% gray-zone. In addition, 13.4% (n = 23) of 172 patients were found to have both RSV and adenovirus coexistence. There was a statistically significant difference (P < 0.05) in terms of gender in viral positive patients.
Conclusion:
In this thesis study, the prevalence of specific immune responses developed in individuals against RSV and adenovirus, which play a role in COPD attacks and exacerbations has been revealed. We suggest that it will be effective to use virus-specific vaccines as a treatment modality for the elimination of viral agents that increase the severity of exacerbations in unvaccinated COPD patients.
Keywords
Chronic obstructive pulmonary disease
Respiratory syncytial virus
Adenovirus
Immunoglobulin G
Seroprevalence
Enzyme-linked immunosorbent assay
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is an increased chronic inflammatory response of the airways and lungs exposed to harmful gasses and particles.[1,2] The main risk factor for COPD is to use tobacco. In addition to the use of tobacco and tobacco products, exposure to harmful particles due to the use of fuel with biocide, exposure to grain dust during agricultural operations, exposure to the vapor of metal corrosive chemical agents used in industry and industry, exposure to ammonia and refrigerant gasses, air pollution, genetic susceptibility, and poor nutrition cause COPD development.[3-5]
In COPD patients, microbial colonization, lung inflammation, systemic inflammation, loss of lung parenchyma, and chronic airflow limitations are observed with increased susceptibility to infection in the lung.[6,7] COPD is characterized by exacerbation. Exacerbation is the most common symptom of the disease, with increased difficulty in breathing, increased cough, and increased sputum.[1,4] Exacerbations are caused by bacterial or viral infections, air pollution, and sudden ambient temperature changes. In this case, respiratory distress and worsened pulmonary gas exchange occur. Ventilation/perfusion imbalance initiates inflammation.[8] Chronic exacerbation in COPD leads to high morbidity, increased frequency of use of health resources and worsening health status of patients , and causes high mortality rate.[9] Respiratory tract viruses entering the body, which can cause upper and lower respiratory tract infections are called respiratory tract viruses.[10] In COPD patients, influenza A and B, picornavirus, respiratory syncytial virus (RSV), parainfluenza, rhinovirus, coronavirus, metapneumovirus, and adenoviruses are known to induce acute exacerbations and worsening the symptoms of the disease.[11-13]
The aim of this study was to determine the seroprevalence of RSV immunoglobulin G (IgG) and adenovirus IgG in COPD patients.
MATERIALS AND METHODS
Serum samples
The one hundred and seventy-two patients over 40 years of age who were followed up with a diagnosis of COPD in the Chest Diseases Polyclinic were included in the study. This study is included in the project supported by the Hitit University BAP unit and was approved by the Noninterventional Clinical Research Ethics Committee (2017/5; 2018/222). Our research was conducted in accordance with the World Medical Association (Declaration of Helsinki). Blood samples and nasopharyngeal swab samples were collected from patients during their routine examinations in the fall and winter.
Determination of RSV and adenovirus IgG levels
To determine the viral positivity, the ELISA method was preferred with the appropriate ELISA kits (DRG Instruments GmbH, Germany) suitable for RSV and Adenovirus.
Serum samples were diluted before the study. For this, 1000 ratio of Sample Diluent was added to 1 patient’s serum. After dilution, the samples were waited for 15 min. The required number of microtiter strip wells was placed in the plate. The 1st well (A1) is left blank for the substrate blank. 100 microliters of Negative control were added to the next 2 wells (B1 and C1). 100 microliters of cutoff control were placed in 2 wells (C1 and D1). 100 microliters of Positive Control were placed in 1-well (E1). 100 microliters of the diluted serum sample were added to each remaining well. Cover the plate with foil and incubate at 37°C for 60 min. Once the incubation period was complete, the plate was shaken. The contents in the wells were washed 5 times with 300 microliters of washing solution per well. After washing, the plate was hit sharply on the absorbent paper and the remaining droplets were removed. 100 microliters of enzyme conjugate were added to each well, except for A1 well. The test plate was incubated for 30 min at room temperature (20–25°C) protected from sunlight. Once the incubation period was complete, the plate was shaken. The contents in the wells were washed 5 times with 300 microliters of washing solution per well again. 100 microliters of Substrate Solution were added to each well and incubated for 15 min at room temperature (20–25°C) protected from sunlight. 100 microliters of Stop Solution were added to each well to stop the enzymatic reaction. The color of each well during incubation changed from blue to yellow.
Measurement of the absorbance value of the samples
The absorbance value of the samples in the plate within 30 min after the addition of the stop solution was measured at 450 nanometers and 620 nanometers in the ELISA Reader (Rayto 3100, Germany).
Substrate blank’s absorbance value (A1) must be <0.100.
Negative control cup absorbance value (B1): Must be <0.200.
The absorbance values of the cutoff control wells (C1/D1) should be between 0.350 and 0.900.
The absorbance value (E1) of the positive control well should be between 0.650 and 3.000.
Calculation of results
The mean cutoff value (CO) is calculated by taking the arithmetic average of the absorbance values measured first, for example, (0.59 + 0.61)/2 = 0.60.
Positive result
The mean absorbance value of the patient should be >10% of the CO value. (Average Optical Densitypatient >1.1 × CO).
Gray zone
If the patient’s serum absorbance value reading the test result shows the absorbance value between 10% and 10% of the CO value, the result is calculated as a gray zone (0.9 × CO 1 average optical densitypatient ≥1.1 × CO). After 2–4 weeks, these samples are taken again, and the experiment is repeated. If the result is again in the gray zone, the patient is positive.
Negative outcome
Patient samples with an absorbance value <10% below the CO value are negative (Average Optical Densitypatient <0.9 × CO).
Statistical analysis
Statistical analysis of the study was performed using IBM SPSS Statistics 22.0 (IBM Co., Armonk, NY, USA) program. Results are presented as mean ± standard deviation. P < 0.05 values were considered statistically significant. Chi-square and Fisher’s exact test were used to compare qualitative data.
RESULTS
In our study, in the autumn and winter seasons of COPD exacerbations, serum samples of 172 patients over 40 years of age who were diagnosed with COPD and were treated were used. Sixty-five (37.8%) of the patients were female and 107 (62.2%) were male.
RSV IgG results
In the first RSV IgG study, 40.7% (n = 70) of 172 serum samples included in the study were RSV IgG positive, 49.4% (n = 85) were RSV IgG negative. In the first experiment, 9.9% (n = 17) of the samples were in the gray zone. In the repeated study, three of these samples were found to be positive. As a result, 42.5% (n = 73) of the samples were positive, 49.4% (n = 85) were identified as RSV IgG negative, and 8.1% (n = 14) as gray zone. When the RSV IgG data were evaluated in terms of sex, 35.5% (n = 38) of 107 male patients and 53.8% (n = 35) of 65 female patients were positive for RSV IgG. In addition, 54.2% (n = 58) of male patients and 41.5% (n = 27) of female patients were found to be RSV IgG negative.
According to RSV IgG data, 78.5% (n = 11) of the values in the gray zone belonged to male and 21.5% (3) belonged to female patient [Table 1]. There was a statistically significant difference between the sexes (male/female) in RSV positive patients (P < 0.05).
| RSV IgG status | Male (n=107, %) | Female (n=65, %) | Total (n=172) |
|---|---|---|---|
| RSV positive patients | 38 (35.5) | 35 (53.8) | 73 |
| RSV negative patients | 58 (54.2) | 27 (41.5) | 85 |
| Gray zone | 11 (10.3) | 3 (4.6) | 14 |
RSV: Respiratory syncytial virus, IgG: Immunoglobulin G
Adenovirus IgG results
In the first adenovirus IgG study, 26.7% (n = 46) of the samples included in the study were identified as adenovirus IgG positive and 61.6% (n = 106) were determined as adenovirus IgG negative. In addition, 11.6% (n = 20) of the samples were in the gray zone. In the repeated study, six of these samples were positive for adenovirus IgG. As a result, 30.2% (n = 52) of the samples were positive, 61.6% (n = 106) negative, and 8.2% (n = 14) as gray zone.
In terms of gender, 30% (n = 32) of 107 male patients and 30.8% (n = 20) of 65 female patients were found to be positive for adenovirus IgG. In addition, 59.8% (n = 64) of the male patients and 64.6% (n = 42) of the female patients were negative for adenovirus IgG. Of the values in the gray zone, 78.5% (n = 11) were male, and 21.50% (n = 3) belonged to female patients [Table 2]. There was a statistically significant difference in sex (male/female) in adenovirus positive patients (P < 0.05).
| Adenovirus IgG status | Male (n=107, %) | Female (n=65, %) | Total (n=172) |
|---|---|---|---|
| Adenovirus positive patients | 32 (30.0) | 20 (30.8) | 52 |
| Adenovirus negative patients | 64 (59.8) | 42 (64.6) | 106 |
| Gray zone | 11 (10.2) | 3 (4.6) | 14 |
IgG: Immunoglobulin G
Results of coexistence of RSV and adenovirus
Both RSV and adenovirus positivity were found in 23 (13%) of 172 patients. About 60.9% (n = 14) of the positive specimens were male and 39.1% (n = 9) were female patients [Figure 1].
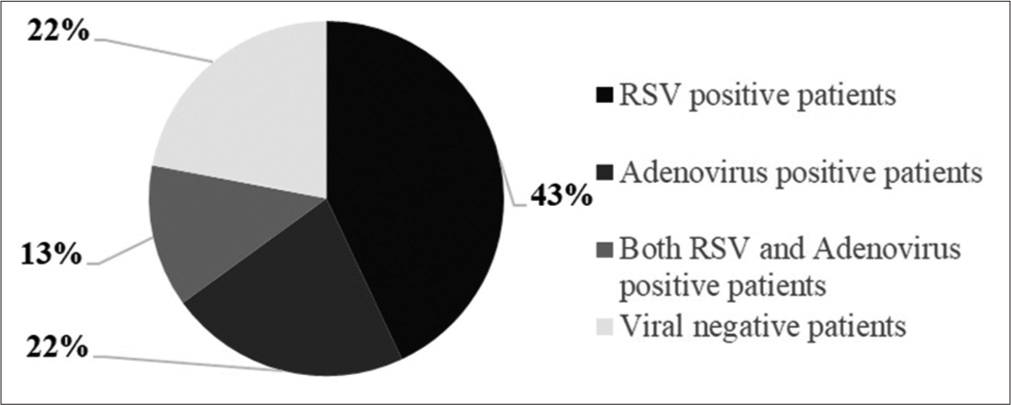
- Respiratory syncytial virus (RSV) and adenovirus positivity distributions.
DISCUSSION
COPD is the most common chronic airway disease in adults, with no progression of airway restriction.[14,15] Although the main etiologic agents associated with COPD are cigarette smoking and biomass exposure, respiratory tract infections play an important role in both the pathogenesis of stable COPD and acute exacerbations.[16]
In our study, in the autumn and winter seasons, when COPD exacerbations were common, serum samples of 172 patients over 40 years of age who were diagnosed with COPD and were in treatment were used. Studies have shown that respiratory tract viruses can be isolated from samples such as bronchoalveolar lavage, nasopharyngeal aspirate, nasopharyngeal swab, sputum, and bronchoalveolar lavage, while serum samples are used to determine changes in the immune response in the patient.[14,17-19] In our study, it was preferred that the IgG antibodies, which are specific against the viral factor in the patient’s serum samples and that has a longer half-life than IgM, were determined by the ELISA method.
Our study was performed with serum samples obtained in the autumn and winter seasons, especially when COPD exacerbations are more common.[13] Of the 172 specimens included in the study, 73 (42.4%) had RSV IgG positivity and 52 (30.2%) had adenovirus IgG positivity. Coinfection (RSV + Adenovirus) was detected in 23 (13.4%) of the patients also. About 60.9% (n = 14) of the samples with coinfection were male and 39.1% (n = 9) were female patients. Coinfection is not very common due to interference in both viruses and bacteria. However, because our study relies on the investigation of the presence of IgG in serum samples, it suggests that individuals with coinfection are successive or have had a previous infection. This can be clarified by studies focused on the viral agent to be carried out by molecular methods. In our study, a statistically significant difference was found in sex (male/female) in both COPD patients and RSV positive patients (P < 0.05). However, there was no statistically significant difference in adenovirus positivity (P >0.05). COPD disease is more common in males than females due to high exposure to risk factors. However, it was determined that the presence of viral agent was higher in female patients.
Tan et al. examined patients with COPD and asthma in endotracheal aspirate and sputum samples and in the study, reported that the most influenza virus was found. In the study, it has been reported that picornavirus, adenovirus, and influenza A and B type viruses were encountered.[20] In another study, scientists studied viral and bacterial infectious agents in sputum samples from 64 COPD patients. In the study, bacteria or viruses were detected in 78% of the patients in COPD exacerbation period. Lung function loss was also reported in these patients. It has also been reported that the duration of hospitalization of coinfected individuals is longer. The presence of increased neutrophils and eosinophils in the sputum of individuals with viral or bacterial infection was recorded.[19]
They reported that exacerbations of COPD were triggered by bacterial or viral pathogens, the infectious agents. It is emphasized that these pathogens cause an increase in inflammatory markers in the individual and cause the formation of exacerbation table.[21] Hutchinson et al., reported that viruses causing respiratory tract infection accompanied by acute exacerbation in COPD patients. Nasopharyngeal swab samples from 92 patients with a mean age of 72 years were investigated with multiplex polymerase chain reaction (PCR) and atypical pneumonia serology tests. It has been reported that 148 exacerbations were recorded during the 99-week surveillance period and the viral isolation rate in the samples increased 11-fold during exacerbation. In the study, it has been reported that the viral agents of picornavirus, influenza A, parainfluenza type 1, 2, 3, RSV, and adenovirus species were isolated from the patients.[14] Nichols et al. reported that respiratory viral infections cause seasonal colds, bronchiolitis, acute otitis, sinusitis, croup, community-acquired pneumonia, and exacerbations of both COPD and asthma and are a major cause of morbidity and mortality. The researchers reported that RSV and adenovirus infections caused specific infections.[22] They suggest that in COPD, treatment strategies should be determined according to bacterial-viral infections and the immune response that develops against these factors. Viral infection was found in 41.9% of COPD patients with acute exacerbation and in 33.5% of COPD patients with pneumonia. Influenza virus is the most common virus type in the exacerbation group. Coronavirus has been reported in the COPD patient group with concomitant pneumonia. Only viral infection, infection only with bacterial infection, and both viral and bacterial infection (concomitant infection) groups compared the highest rate of congestion in the hospital reported the mortality rate.[23] Kwak et al. performed a study to evaluate the prevalence of respiratory tract infection in COPD exacerbations, to find factors related to susceptibility to viral infections, and to compare the effects of viral pathogens on non-virus pathogens. In their study, patients treated in hospitals for more than 2 years were examined. As a result of the study, nasopharyngeal swab samples were examined by multiplex PCR technique. The most isolated rhinovirus (38.8%) species was isolated. The presence of viral agents, such as RSV, coronavirus, influenza A, parainfluenza, adenovirus, and metapneumovirus, have also been reported. It is reported that the presence or absence of viral agents in the patient does not affect the severity of exacerbation, whereas the rate of viral infection in the female population in the sample is reported to be higher.[24] The data obtained in our study show that the disease is more common in the male population in our sample.
Greenberg et al. in their study, followed for 26–35 months and influenza vaccination in elderly patients with COPD and investigated the importance of viral infection. In this cohort study, 27% reported the presence of viral infection in the control group (i.e., in a group of non-COPD patients). Of COPD patients, 44% stated that they cause viral respiratory tract diseases. Among the factors, picornaviruses, parainfluenza viruses, and coronavirus viruses have been reported to be the most frequent agents.[25] In our samples, the incidence of RSV virus was higher than the incidence of adenovirus. It is reported that RSV infection is the most common cause of respiratory infections in the respiratory tract, and it is reported that it is very important to detect the patients receiving care, especially in newborns. Because of this viral pathogen, it is stated that the most accurate diagnosis and treatment of respiratory disease is prevented by the physician.[26] Researchers noted that respiratory tract viruses are in the lower respiratory tract and prepare the ground for secondary bacterial infections in patients with stable COPD. In their study to determine the clinical effects of RSV, Kim et al. compared samples taken from two different groups (COPD patients with acute exacerbation and COPD patients with concomitant pneumonia) using the RT-PCR method.[23] In our study, viral positivity was evaluated based on demonstration of IgG molecule synthesized specific to the viral agent from the body of the individual.
Because RSV and Adenovirus agents may be among the causes of the pathogenesis of the exacerbation phase of the disease, we discussed this research in the name of choosing more palliative treatment methods for the treatment of the disease (influenza vaccination, conscious use of antibiotics, and selection of specific treatment modality).[27] Our future work will proceed in this direction.
Viral infection inhibits NK cell responses in the lung, leading to increased susceptibility to subsequent bacterial superinfection.[28] In one study, the efficacy of influenza vaccine on a sample of six patients with COPD and others treated with chronic lung disease was investigated. COPD patients who received influenza vaccine compared to the placebo-treated group were reported to have a significantly reduced recurrence of exacerbation within 3–4 weeks after administration. Fluid vaccination is a supportive treatment for patients treated for COPD. In a concrete way, influenza virus-induced infection triggers acute exacerbation in COPD patients, leads to worsening of symptoms and increases mortality and morbidity.[29] In our study, we suggest that vaccination as a preventive treatment will be effective if it is specific to the agent to minimize the adverse effects of the disease.
The prevalence of COPD varies across countries and demographically. This prevalence increases with the frequency of indoor air pollution, occupational exposure, and tobacco smoke. The aging of the current population accelerates this increase.[30] Global Burden of Disease Study 2010 data show that the life of 2.9 million individuals per year ended due to COPD. COPD, the third cause of death among all deaths worldwide, covers 5.5% of all deaths. This is an indicator of high mortality with early mortality, increased mortality rates, and increased health expenditures.[31,32] Among the ten leading causes of death in the world between 2002 and 2030, their ranking in the cause of death was ranked 5th in COPD in 2002, and this rank is expected to be the 6th in 2030.[30] BREATHE study (Observational Cross-sectional epidemiology study in COPD in the Middle East region) is a study made to evaluate the prevalence of COPD symptoms in 11 countries in Africa and the Middle East region. Tunisia, Egypt, Jordan, Morocco, Pakistan, Saudi Arabia, Lebanon, Syria, United Arab Emirates, Algeria, and Turkey are discussed in the study of the incidence and prevalence of COPD. According to research data, it was reported that the prevalence of COPD in Turkey is 42.2% and 3 million individuals have COPD. Our country is the third most common country in terms of countries where the study is conducted.[33]
In this study, the prevalence of specific immune responses against RSV and adenovirus, which play a role in COPD exacerbations has been demonstrated. We suggest that it will be effective to use virus-specific vaccines as a treatment modality for the elimination of viral agents that increase the severity of exacerbations in unvaccinated COPD patients.
CONCLUSION
In this study, the prevalence of specific immune responses against RSV and adenovirus, which play a role in COPD exacerbations has been demonstrated. We suggest that it will be effective to use virus-specific vaccines as a treatment modality for the elimination of viral agents that increase the severity of exacerbations in unvaccinated COPD patients.
Authorship contributions
Sertac Aslan: Medical practices; Gulcin Alp Avcı, Asiye Asli, Emniyet Sert, Sertac Aslan: Concept; Gulcin Alp Avcı, Asiye, Asli Emniyet Sert: Design; Gulcin Alp Avcı, Asiye Asli, Emniyet Sert, Sertac Aslan: Analysis or Interpretation; Gulcin, Alp Avcı, Asiye Asli Emniyet Sert: Literature search; Gulcin, Alp Avcı, Asiye Asli Emniyet Sert: Writing.
Ethical approval
The study is approved by Hittite University Ethics Committee, Turkey (2017/5;2018/2022).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
This study is included in the project supported by Hittite University BAP unit (FEF19001.19.001).
References
- The impact of anxiety and depression in chronic obstructive pulmonary disease. Adv Respir Med. 2023;91:123-34.
- [CrossRef] [PubMed] [Google Scholar]
- Triple therapy in COPD: Can we welcome the reduction in cardiovascular risk and mortality? Front Med (Lausanne). 2022;9:816843.
- [CrossRef] [PubMed] [Google Scholar]
- Role of lifestyle in the development of chronic obstructive pulmonary disease: A review. Lung India. 2008;25:95-101.
- [CrossRef] [PubMed] [Google Scholar]
- Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21 century. Int J Environ Res Public Health. 2009;6:209-24.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors and pathophysiology of chronic obstructive pulmonary disease (COPD) J Assoc Physicians India. 2012;60(Suppl):17-21.
- [Google Scholar]
- Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper [published correction appears in. Eur Respir J. 2004;23:932-46.
- [CrossRef] [PubMed] [Google Scholar]
- Combined exposure to bacteria and cigarette smoke resembles characteristic phenotypes of human COPD in a murine disease model. Exp Toxicol Pathol. 2015;67:261-9.
- [CrossRef] [PubMed] [Google Scholar]
- ABC of chronic obstructive pulmonary disease pathology, pathogenesis, and pathophysiology. Br Med J. 2006;332:1202-4.
- [CrossRef] [Google Scholar]
- Impact of exacerbations on COPD. Eur Respir Rev. 2010;19:113-8.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory viruses In: Encyclopedia of microbiology. Beltsville, MD: Plant Sciences Institute; 2009. p. :500-18.
- [CrossRef] [Google Scholar]
- The classification of viruses infecting the respiratory tract. Paediatr Respir Rev. 2003;4:84-90.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory viral and bacterial exacerbations of COPD-the role of the airway epithelium. Cells. 2022;11:1416.
- [CrossRef] [PubMed] [Google Scholar]
- Importance of viral and bacterial infections in chronic obstructive pulmonary disease exacerbations. J Clin Virol. 2009;46:129-33.
- [CrossRef] [PubMed] [Google Scholar]
- A community-based, time-matched, case-control study of respiratory viruses and exacerbations of COPD. Respir Med. 2007;101:2472-81.
- [CrossRef] [PubMed] [Google Scholar]
- A one-year prospective study of infectious etiology in patients hospitalized with acute exacerbations of COPD and concomitant pneumonia. Respir Med. 2008;102:1109-16.
- [CrossRef] [PubMed] [Google Scholar]
- Lung microbiology and exacerbations in COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:555-69.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of a quantitative real-time PCR for the detection of respiratory syncytial virus in pulmonary diseases. Eur Respir J. 2003;21:944-51.
- [CrossRef] [PubMed] [Google Scholar]
- IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J Immunol. 2004;172:7603-9.
- [CrossRef] [PubMed] [Google Scholar]
- Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114-21.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of respiratory viruses in patients hospitalized with near-fatal asthma, acute exacerbations of asthma, or chronic obstructive pulmonary disease. Am J Med. 2003;115:272-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest. 2006;129:317-24.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory viruses other than influenza virus. Clin Microbiol Rev. 2008;21:274-90.
- [CrossRef] [PubMed] [Google Scholar]
- Different pattern of viral infections and clinical outcomes in patient with acute exacerbation of chronic obstructive pulmonary disease and chronic obstructive pulmonary disease with pneumonia. J Med Virol. 2016;88:2092-9.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and risk factors of respiratory viral infections in exacerbations of chronic obstructive pulmonary disease. Tohoku J Exp Med. 2016;240:131-9.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:167-73.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory syncytial virus infection in children In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023.
- [Google Scholar]
- Bacterial-viral load and the immune response in stable and exacerbated COPD: Significance and therapeutic prospects. Int J Chron Obstruct Pulmon Dis. 2016;11:445-53.
- [CrossRef] [PubMed] [Google Scholar]
- Influenza infection leads to increased susceptibility to subsequent bacterial superinfection by impairing NK cell response in the lung. J Immun. 2010;184:2048-56.
- [CrossRef] [PubMed] [Google Scholar]
- Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;1:CD002733.
- [CrossRef] [Google Scholar]
- Epidemiology and risk factors of chronic obstructive pulmonary disease in Suzhou: A population-based cross-sectional study. J Thorac Dis. 2020;12:5347-56.
- [CrossRef] [PubMed] [Google Scholar]
- Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-128.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and correlates of chronic obstructive pulmonary disease and chronic respiratory symptoms in rural southwestern Uganda: A cross-sectional, population-based study. J Glob Health. 2019;9:10434.
- [CrossRef] [PubMed] [Google Scholar]
- Distrubution of COPD releated symptoms in the Middle East and North Africa: Result of the BREATHE study. Respir Med. 2012;106:25-32.
- [CrossRef] [PubMed] [Google Scholar]







