Translate this page into:
An unusual case of rectal schwannoma: A case report
*Corresponding author: Safa Alshaikh, Department of Pathology, Salmaniya Medical Complex, Manama, Bahrain. dr_salshaikh@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Alshaikh S, Almuqamam A, Mubarak A. An unusual case of rectal schwannoma: A case report. Indian J Med Sci. 2024;76:140-3. doi: 10.25259/IJMS_64_2024
Abstract
Rectal schwannomas, although exceedingly rare, represent an intriguing and often perplexing diagnostic challenge in the field of gastroenterology. Here, we are presenting an interesting case of rectal mass in a 66-years-old Bahraini male who has a known case of irritable bowel and presented with symptoms of fullness in the right iliac fossa region accompanied by recurrent episodes of constipation. A large upper rectal mass was identified in imaging studies; however, obtaining a biopsy was difficult using sigmoidoscopy three times; hence, the patient eventually underwent an exploratory laparoscopy with colectomy. Pathologist confirmed the diagnosis of schwannoma. This captivating case study unravels the enigma surrounding a unique encounter with rectal schwannoma. In addition, we represent an enlightening case that widens our differential diagnosis in investigating mesenchymal tumors of the gastrointestinal tract with emphasis on its clinical presentation, diagnostic nuances, and therapeutic consideration.
Keywords
Schwannoma
Laparoscopy
Irritable bowel
INTRODUCTION
Schwannomas are rare neoplasms that arise from the Schwann cells of the peripheral nervous system. Schwann cells serve an essential role in encasing peripheral nerves with myelin sheaths. Hence, schwannomas can arise in different areas within the body as either part of a genetic syndrome like neurofibromatosis Type 2, in which the vestibular nerve of the ears is affected bilaterally, or sporadically as a histopathological diagnostic finding. Among all gastrointestinal tract schwannomas, colorectal schwannomas tend to be an extremely infrequent entity with an incidence of <5%.[1] Correlating with the clinical findings, gross description, microscopic analysis, and immunohistochemical staining is the cornerstone for a definitive diagnosis of this rare pathology. This case report will discuss a case of schwannoma of the rectum accompanied by a literature review.
CASE REPORT
A 66-year-old male patient, a known case of irritable bowel syndrome, hypertension, and chronic varicose veins, presented to the emergency room with a history of chronic heaviness in the left iliac fossa and “on and off” constipation for the last few months.
An oral, rectal, and IV contrast-enhanced computed tomography scan of the chest, abdomen, and pelvis primarily shows an exophytic bilobed soft-tissue lesion involving the upper rectum with intraluminal extension. Small volume lymph nodes surrounding the superior rectum are noted. No signs of bowel obstruction are detected. No free fluid or pneumoperitoneum was demonstrated. The rest of the pelvic organs are also unremarkable. The abdominal vasculature was not significant apart from the minor atherosclerotic disease of the aorta and its branches.
A multiplanar, multisequential pre- and post-contrast enhanced magnetic resonance imaging pelvis showed well-defined, polypoidal soft-tissue mass, arising from the upper rectum. The lesion has increased in size, currently measuring 5 cm from anterior to posterior × 6 cm from medial to lateral × 6.8 cm from superior to inferior, compared to 4.5 cm × 5 cm × 5.8 cm respectively, previously. The lesion is isointense on the T1 wave, Iso to slightly hyperintense on the T2 wave, and shows mild diffusion restriction. Post-contrast, it shows mild homogenous enhancement. The lesion is exophytic, bulging anteriorly more to the left, with an intraluminal component causing focal narrowing of the upper rectum, noted approximately 13.5 cm proximal to the anal verge. Multiple small-sized lymph nodes were noted along the superior rectal vessels measuring up to 0.6 cm in short axis, these are largely stable. No significant mesorectal and pelvic lymphadenopathy by size criteria. The mesorectal fascia and the pelvic peritoneal reflections are intact. The urinary bladder is compressed posteriorly by the described rectal mass, otherwise unremarkable. Given the appearance and temporal stability, findings favor gastrointestinal stromal tumor (GIST).
Three separate attempts of colonoscopic biopsies were obtained that were non-representative, showing only colonic mucosa with few dilated crypts and mild chronic non-specific inflammation. No dysplastic changes or infiltration were noted in the deeper levels studied.
The patient, later on, underwent exploratory laparotomy comprising resection of the lesion and stoma creation. Intraoperatively, a 10 × 5 cm exophytic bilobed mass in the rectum is excised. No mesenteric lymph nodes noted. The removed specimen was sent for histopathology.
On gross examination, the specimen is received fixed in formalin, labeled with the name of the patient, medical record number as (rectum with mass), and consists a segment of rectum measuring 15.5 cm in length, 3 cm in diameter, and a total weighing 356 g. The serosa was unremarkable. On cutting open the specimen, a well-defined mass was noted, measuring 7 × 7 × 6 cm, and was surrounded by erythematous mucosa. The mass is 1.5 cm away from the nearest distal resection margin and 7 cm away from the proximal resection margin. Multiple lymph nodes were also identified in the pericolic fat.
On microscopic histopathological assessment, sections show congested colonic mucosa with an underlying well-circumscribed tumor, composed of alternating hypercellular areas with myxoid hypocellular areas. The tumoral cells depict nuclear palisading representing verocay bodies. The cells of the tumor were bland-looking spindle to ovoid in shape, with elongated nuclei, dense chromatin, and ill-defined cytoplasm. Thickened hyalinized blood vessels, prominent collagen bands, degenerated nuclear atypia, and prominent lymphoid rim are seen. Mitotic figures are rare and no necrosis is identified. Multiple reactive lymph nodes are noted as well [Figure 1a-c] all the margins are free of tumor.
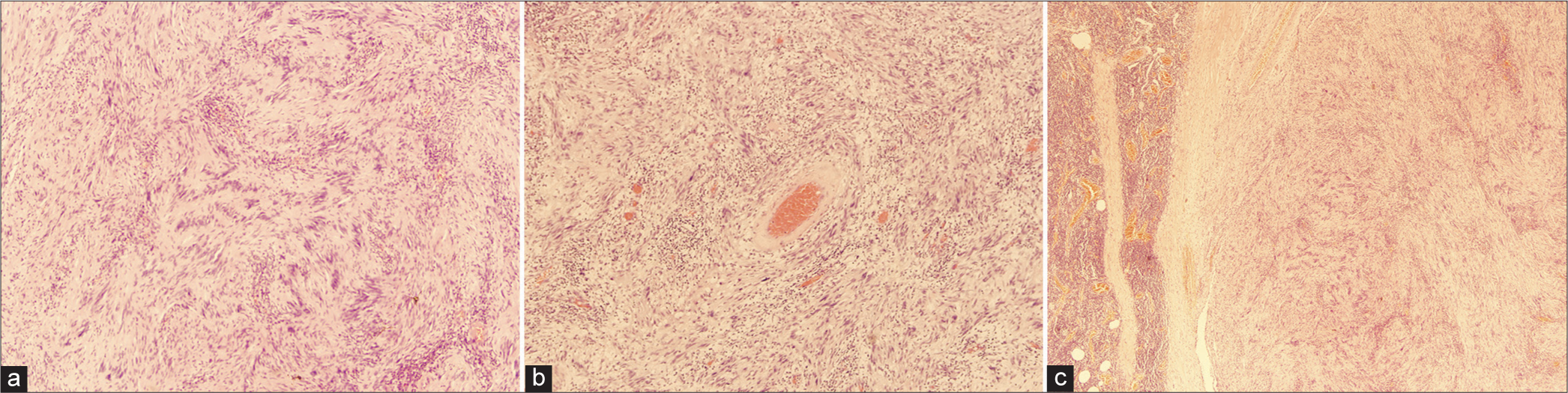
- (a) Nuclear palisading around the fibrillary process (Verocay body) seen in cellular areas ×40. (b) Thickened hyalinized blood vessel wall ×40. (c) Well-circumscribed schwannoma with rim of lymphocytes ×40.
Immunohistochemical stains reveal positivity of the tumor cells for S-100 [Figure 2], while negative for DOG-1, CD117, CD34, SMA, Desmin, ALK-1. Proliferation index (Ki-67) is low.

- S-100 stain showing diffuse positivity ×40.
DISCUSSION
Although mesenchymal tumors of the gastrointestinal tract are considered a rare entity, gastrointestinal schwannomas are even scarcer. Schwannomas are benign tumors that arise from the differentiate nerve sheath cells. They are slow-growing tumors and usually solitary. The exact pathophysiology of schwannoma is unclear, as a majority of cases occur spontaneously[2] while few cases occur in a setting of genetic syndromes such as neurofibromatosis Type 2 or Carney complex.[3]
Schwannomas can occur in any age with equal gender predilection, and the most common age group is between 20 and 40 years.[4] The most common sites are the extremities, head and neck region, and posterior mediastinum. Other visceral organs from which schwannoma may rise include gastrointestinal tract organs, thyroid glands, and very rarely in the vulvar area.[4]
The tumor is mainly asymptomatic and presents as a painless slow-growing mass.
Imaging studies show well-circumscribed mass, with or without cystic degeneration, hemorrhage, and calcifications. However, no evidence of surrounding invasion should be seen in radiological studies.
The gold standard for diagnosis is histopathologic examination by hematoxylin and eosin morphology identification as well as immunohistochemistry. Schwannomas under the microscope will appear a biphasic tumor, composed of bland spindle cells with hypo and hypercellular areas representing what is known as Antoni A and Antoni B areas. Another characteristic histological feature is the presence of verocay bodies, which are fibrillary processes surrounded by nuclear palisading. Ancient changes may also be seen, comprising degenerative nuclear atypia, cystic degeneration, and hemorrhage. The tumor cells should be positive for S-100, podoplanin, calretinin, and Sox-10 on immunohistochemical markers.
As for colonic and rectal schwannomas, a clinicopathological and immunohistochemical analysis of twenty cases found a mild propensity to occur in women compared to men with an average age of presentation of 65 years of age.[5] This median age is congruent with this case’s patient age. Another similar study that reviewed twenty-four cases of gastrointestinal tract schwannomas found that twenty-three of them arise from the stomach and only one case in the ascending colon.[6] This gastric-preferred location for gastrointestinal schwannomas has been documented in the literature.
A retrospective analysis of 78 cases of gastrointestinal schwannomas found the median tumor size to be 3.63 ± 2.03 cm with a wide range of 0.3–10 cm with as few as two rectal cases only.[7] The tumor in the above-described case report was 7 cm in size, which is almost double the average size but within the range seen in the literature. Due to the indolent growth pattern and variability in the location and size of the schwannoma, the presenting symptoms tend to be non-specific and range from being asymptomatic to upper and lower gastrointestinal symptoms. In a case report that reviewed the cytology results in a gastric schwannoma, found that endoscopic ultrasound-guided fine-needle aspiration can yield a smear that is mostly rich in small reactive lymphocytes.[8] This is probably due to the prominent lymphoid cuff that tends to circumscribe the tumor. This might explain the initial non-diagnostic biopsy findings seen in the above-mentioned patient, which may obscure the tumor and mistakenly lead histopathologists to suspect a lymphoid lesion instead. Furthermore, the risk of tumor spread and rupture after a biopsy should be considered in every case.
Resecting the tumor surgically is considered the gold standard of treatment modalities. Alternatively, less invasive endoscopic resection can be considered depending on the tumor size and locality. If the tumor’s surgical margins are free, an excellent over 95% 5-year survival rate can be seen.[9] A study of 33 gastrointestinal schwannoma found that this non-encapsulated, bland spindle cell tumor is usually strongly positive for S100 protein and frequently negative for CD117, Alpha-smooth muscle actin, and desmin.[10] In our case, the immunohistochemical markers are concordant with the published cases.
Most literature agreed that the course of schwannomas is indolent, though exceptional cases are reported to have malignant transformation.[11] A 20-year cohort study from 2000 to 2020 to assess the natural history of gastrointestinal schwannomas, concluded to advise patients, once diagnosed, to remain up to date with other routine cancer screening guidelines and to stick to their scheduled follow-up to detect any malignant transformation or recurrence as early as possible.[12]
CONCLUSION
Schwannoma of the gastrointestinal tract is an infrequent mesenchymal lesion. Although it is a histopathological diagnosis, correlating with the clinical history and radiological findings as well as immunohistochemical profile is the cornerstone for diagnosing such challenging entities, as many differential diagnoses may be included in the differential of gastrointestinal mesenchymal tumors, on top of them is GIST.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Schwannoma of the colon and rectum: A systematic literature review. World J Surg Oncol. 2018;16:125.
- [CrossRef] [PubMed] [Google Scholar]
- Vestibular schwannoma In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023.
- [Google Scholar]
- An unusual case of vulvar schwannoma. World J Surg Oncol. 2015;13:139.
- [CrossRef] [PubMed] [Google Scholar]
- Schwannomas in the colon and rectum: A clinicopathologic and immunohistochemical study of 20 cases. Am J Surg Pathol. 2001;25:846-55.
- [CrossRef] [PubMed] [Google Scholar]
- Benign schwannoma of the gastrointestinal tract: A clinicopathologic and immunohistochemical study. Hum Pathol. 1988;19:257-64.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathological characteristics of gastrointestinal schwannomas: A retrospective analysis of 78 cases. Front Oncol. 2022;12:1003895.
- [CrossRef] [PubMed] [Google Scholar]
- Cytologic findings of gastric schwannoma: A case report. Diagn Cytopathol. 2014;42:177-80.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical characteristics and surgical management of gastrointestinal schwannomas. Biomed Res Int. 2020;2020:9606807.
- [CrossRef] [PubMed] [Google Scholar]
- Schwannoma of the gastrointestinal tract: A clinicopathological, immunohistochemical and ultrastructural study of 33 cases. Histopathology. 2006;48:536-45.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous transformation of vestibular schwannoma into malignant peripheral nerve sheath tumor. Asian J Neurosurg. 2018;13:810-3.
- [CrossRef] [PubMed] [Google Scholar]
- Natural history of gastrointestinal schwannomas. Endosc Int Open. 2022;10:E801-8.
- [CrossRef] [PubMed] [Google Scholar]







