Translate this page into:
Assessment of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, and mean platelet volume levels in oral squamous cell carcinoma and their correlation with histological grading and TNM staging
*Corresponding author: Riya Verma, Department of Oral Pathology and Microbiology, Maulana Azad Institute of Dental Sciences, New Delhi, India. riyav1112@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Verma R, Kumar P. Assessment of neutrophillymphocyte ratio, platelet-lymphocyte ratio, and mean platelet volume levels in oral squamous cell carcinoma and their correlation with histological grading and TNM staging. Indian J Med Sci. 2025;77:6-10. doi: 10.25259/IJMS_119_2024
Abstract
Objectives:
The inflammatory response of the host plays a role in the prognosis of oral squamous cell carcinoma (OSCC). We studied the levels of neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), and mean platelet volume (MPV) in OSCC patients and correlated them with TNM staging and histopathological grade to assess their role in prognosis.
Materials and Methods:
The study included 30 cases of OSCC and 30 controls. The mean NLR, PLR, and MPV of patients with OSCC were compared to the control group. Differences between different variables were analyzed using one-way analysis of variance test and unpaired t-test. Spearman’s rho test was used to determine the correlation between NLR, PLR, and MPV values in OSCC cases.
Results:
Mean PLR and NLR were higher, and mean MPV was lower in the OSCC group than in the control group. There was a statistically significant relationship between PLR and clinical TNM stage of cancer. Furthermore, there was a statistically significant relationship between PLR and NLR and histological stages of cancer. A statistically significant positive correlation between NLR and PLR was also observed.
Conclusion:
NLR and PLR, two inflammatory blood markers, have a significant prognostic impact on OSCC. MPV levels were not as important in predicting prognosis in OSCC as NLR and PLR. PLR and NLR are simple to incorporate into medical care and, when combined with other prognostic indicators, can help in the prognosis of OSCC.
Keywords
Mean platelet volume
Neutrophil-lymphocyte ratio
Oral squamous cell carcinoma
Platelet-lymphocyte ratio
Inflammatory response
INTRODUCTION
Oral squamous cell carcinomas (OSCCs) are malignant neoplasms that commonly affect adults and the elderly. It is ulcerated and has an elevated rolled border around a necrotic central area. The most common sites of occurrence are buccal mucosa and tongue.[1] OSCC is the eighth most common cancer globally and one of the top three in the Indian subcontinent.[2] While males are more affected, females are experiencing an increased number of cases.[3]
Major causes of oral cancer include smoking, drinking, ultraviolet rays, human papillomavirus, candida infections, nutrition deficiencies, and genetics.[4] Early detection can prevent oral cancers. Numerous factors, including age, sex, level of socioeconomic status, race, daily habits, tumor location, size, shape, histology, and treatment modalities, affect the prognosis. The inflammatory response of cancer cells can predict cancer prognosis and impact clinical outcomes in OSCC, influenced by tumor histological properties and the host’s response.[5] Different types of peripheral blood cells, including neutrophils, lymphocytes, monocytes, and platelets, and their ratios, such as neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR), can significantly impact the prognosis of certain tumors, such as esophageal squamous cell carcinoma, cervical carcinoma, and renal carcinoma.[6-8]
Platelet activation is a crucial biological process in cancer occurrence and metastasis, promoting angiogenesis, extracellular matrix disintegration, adhesion molecule release, and growth factors essential for tumor growth and spread.[9,10] Therefore, evaluating platelet index, such as mean platelet volume (MPV) levels, may correlate with prognosis in OSCC.
To the best of our knowledge, no studies are highlighting the prognostic implications of NLR, PLR, and MPV together in OSCC in India, where many people have a habit of chewing tobacco, which leads to oral cancer. Therefore, we conducted the study on the assessment of NLR, PLR, and MPV levels in OSCC and correlated them with histological grading and TNM staging.
MATERIALS AND METHODS
The present study followed a prospective study design. This study was conducted in August–September 2022 in our college. It included 30 patients with OSCC and 30 age- and sex-matched controls. The study was approved by the Institutional Ethical Committee, and patients with other malignancies, pre-operative chemotherapy history, systemic infection, inflammation, or hematological disease were excluded from the study. Controls were selected based on the absence of systemic illness and inflammatory lesions.
The patients were explained about the procedure and informed consent was taken from all the participants in both the groups. A detailed case history was taken. Blood samples of oral cancer subjects were collected preoperatively and a complete hemogram was done using a Sysmex automatic hematology analyzer XT 2000i (Sysmex Corporation, Kobe, Japan). The pre-operative platelet count, absolute lymphocyte count, absolute neutrophil count, and MPV levels were recorded. PLR and NLR values were then calculated. PLR is the absolute platelet count divided by the absolute lymphocyte count. NLR is the absolute neutrophil count divided by the absolute lymphocyte count. The lesion was clinically examined, and staging was done according to the American Joint Committee on Cancer (AJCC) 8th edition. Formalin-fixed paraffin-embedded tissue blocks were used for histopathological examination, with hematoxylin and eosin staining performed on sections. Histopathological grading was done by two oral pathologists using the World Health Organization grading system.
Data were analyzed using the Statistical Package for the Social Sciences software version 26. Data were expressed as mean ± standard deviation. Differences between different variables were analyzed using a one-way analysis of variance (ANOVA) test and an unpaired t-test.
P ≤ 0.05 was considered to be significant. Spearman’s rho test was used to determine the correlation between NLR, PLR, and MPV values in OSCC cases. P ≤ 0.01 was considered to be significant.
RESULTS
The mean age of patients was 49.6 years, and there was male predominance (86.6%). The majority of (63.3%) patients consumed smokeless tobacco; 20% consumed smoked tobacco, while the remaining consumed both smoked and smokeless forms of tobacco. Alveolar mucosa was the most common (40%) site of involvement, followed by buccal mucosa (33.3%), gingiva (10%), labial mucosa (6.6%), lateral border of the tongue (6.6%), and palate (3.3%). Based on AJCC Cancer Staging 8th edition, 60% of patients had TNM Stage IVa cancer, 23.3% were TNM Stage II cancer, 6.6% were TNM Stages III and IVb, and 3.3% were TNM Stage I cancer. There were 17 (56.6%) patients with well-differentiated squamous cell carcinoma (Grade I), 12 (40%) with moderately differentiated OSCC (Grade II), and 1 (3.3%) with poorly differentiated OSCC (Grade III). All other results are mentioned in Tables 1-3.
| Groups | Mean | P-value |
|---|---|---|
| PLR | ||
| Group-1 | 121.536 | 0.002* (<0.05) |
| Group-2 | 72.48 | |
| NLR | ||
| Group-1 | 2.961 | 0.113 |
| Group-2 | 2.431 | |
| MPV | ||
| Group-1 | 11.24 | 0.306 |
| Group-2 | 11.58 | |
NLR: Neutrophil-lymphocyte ratio, PLR: Platelet-lymphocyte ratio, MPV: Mean platelet ratio, Group I: OSCC patient group, Group II: Control group. *: This symbol and bold values means that p value is less than 0.05 and is significant. OSCC: Oral squamous cell carcinoma.
| Histological grade | Mean | SD | F value | P-value |
|---|---|---|---|---|
| PLR | ||||
| Grade I | 123.11 | 48.20 | 3.254 | 0.028* (<0.05) |
| Grade II | 120.67 | 70.06 | ||
| Grade III | 105.00 | 0 | ||
| Controls | 72.48 | 63.70 | ||
| NLR | ||||
| Grade I | 2.911 | 1.09 | 2.843 | 0.046* (<0.05) |
| Grade II | 2.79 | 1.64 | ||
| Grade III | 5.87 | 0 | ||
| Controls | 2.43 | 1.12 | ||
| MPV | ||||
| Grade I | 11.41 | 1.05 | 0.641 | 0.592 |
| Grade II | 10.98 | 1.47 | ||
| Grade III | 11.500 | 0 | ||
| Controls | 11.580 | 1.277 |
NLR: Neutrophil-lymphocyte ratio, PLR: Platelet-lymphocyte ratio, MPV: Mean platelet ratio, SD: Standard deviation, OSCC: Oral squamous cell carcinoma, ANOVA: Analysis of variance. Bold values with ‘*’ symbol means p value is less than 0.05 and is significant.
| Clinical stage | Mean | SD | f-value | P-value |
|---|---|---|---|---|
| PLR | ||||
| Stage I | 239.70 | 0 | 2.954 | 0.020* (<0.05) |
| Stage II | 119.49 | 64.18 | ||
| Stage III | 87.01 | 9.70 | ||
| Stage Iva | 118.88 | 54.27 | ||
| Stage Ivb | 127.9500 | 5.74 | ||
| Controls | 72.4820 | 63.70 | ||
| NLR | ||||
| Stage I | 3.20 | 0 | 0.843 | 0.526 |
| Stage II | 2.88 | 1.47 | ||
| Stage III | 1.99 | 0.23 | ||
| Stage Iva | 3.13 | 1.54 | ||
| Stage Ivb | 2.50 | 0.97 | ||
| Controls | 2.43 | 1.12 | ||
| MPV | ||||
| Stage I | 9.10 | 0 | 1.301 | 0.277 |
| Stage II | 11.42 | 1.08 | ||
| Stage III | 12.35 | 0.49 | ||
| Stage Iva | 11.25 | 1.17 | ||
| Stage Ivb | 10.55 | 2.05 | ||
| Controls | 11.58 | 1.27 |
NLR: Neutrophil-lymphocyte ratio, PLR: Platelet-lymphocyte ratio, MPV: Mean platelet ratio, cTNM: Clinical TNM stage, SD: Standard deviation, OSCC: Oral squamous cell carcinoma, ANOVA: Analysis of variance, Bold values with ‘*’ symbol means p value is less than 0.05 and is significant.
A statistically significant positive correlation (r = 0.623, P < 0.001) was seen between NLR and PLR. PLR was negatively correlated with MPV while NLR was positively correlated but it was not significant statistically [Figure 1].
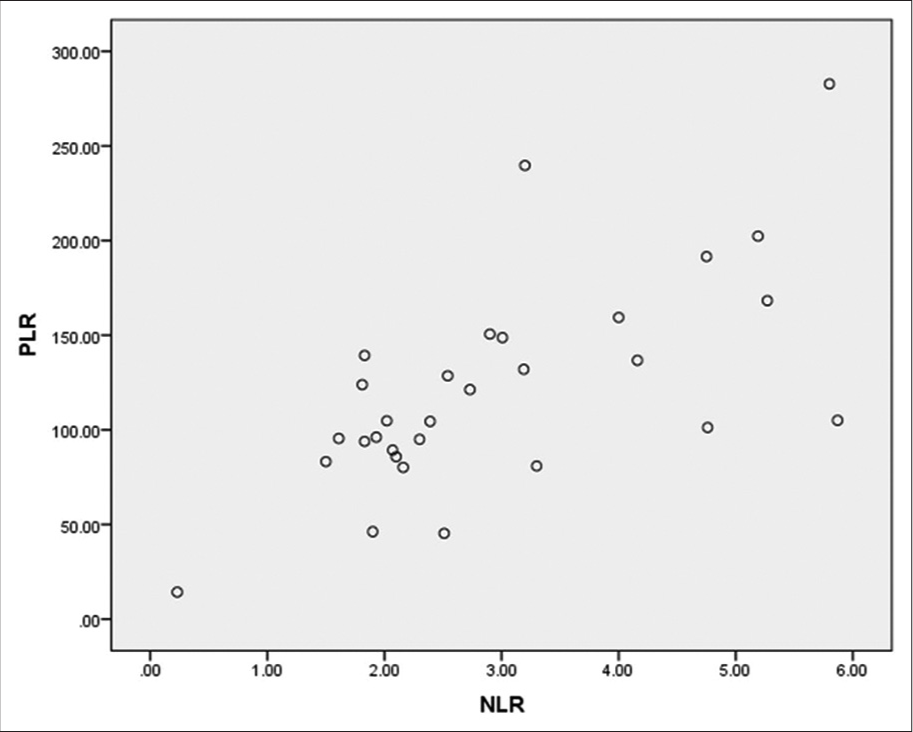
- X-axis – Neutrophil-lymphocyte ratio (NLR), Y-axis – Platelet-lymphocyte ratio (PLR).
The association of clinical characteristics such as size and habit with NLR, PLR, and MPV was also seen, but it was statistically insignificant.
DISCUSSION
Inflammation significantly impacts tumor development and therapy, with key factors including NLR, PLR, and MPV mediating immune-cancer cell dialogue. The present study evaluated the predictive potential of inflammatory markers such as NLR, PLR, and MPV in OSCC patients.
Nikolić et al. found a mean NLR of 3.63 and a mean PLR of 171 for the cancer group and a mean NLR of 2.07 and a mean PLR of 115 for the control group in lung cancer patients.[11] These results were similar to our study in which the mean PLR and NLR were higher in the OSCC group as compared to the control group [Table 1]. It was also seen that the differences between PLR and NLR of lung cancer patients and control groups were statistically significant (P < 0.001). In our study, only PLR showed a significant difference (P = 0.002) using one-way ANOVA. However, in a study by Anand et al., although the mean PLR in OSCC patients was higher than in controls, it was statistically insignificant.[12] High pre-operative PLR and a poor prognosis are associated, but the exact mechanisms are unclear. Sabrkhany et al. highlighted the potential significance of platelets in tumor angiogenesis and proliferation in cancers.[13] According to them, platelets get activated and adhere to the tumor’s endothelial cells causing them to release angiogenic and angiostatic material, promoting angiogenesis.[13] Kim et al. state that platelets expressing P-selectin have the ability to attach to big mucin molecules found on the surface of tumor cells, which have P-selectin binding sites.[14] Tumor-induced platelet activation arcs form when platelets become activated by a tumor through their P-selectin and integrin, causing thrombosis and starting a reciprocating loop.[15] These activated platelets produce a number of growth factors that induce angiogenesis, metastasis, and tumor growth. This vicious cycle leads to the spread of cancer.[15]
In our study, the mean NLR of oral cancer patients was higher than the control group but the findings were not statistically significant (P = 0.113). This is in contrast to a study by Düzlü et al., where NLR was significantly higher in OSCC patients as compared with control groups.[16] This difference in statistical findings could be due to our smaller sample size.
The relationship between NLR and worse outcomes in cancer is not fully understood but may be influenced by neutrophils’ ability to secrete vascular endothelial growth factor, according to a study by Kusumanto et al.[17] For recurrent or metastatic head-and-neck squamous cell carcinoma, NLR derived from a blood sample at the time of the initial diagnosis of the metastatic disease or during recurrence may be a helpful laboratory measure.[18]
Our study demonstrates the statistically significant relationship between PLR and clinical TNM stage of cancer (P = 0.020). The mean PLR values decreased from TNM Stage I to TNM Stage III and then increased in TNM Stages IVa and IVb. This can be due to a lower sample size. Lu et al. found that advanced tumor characteristics, including poorer differentiation, deeper depth, and advanced TNM stages, were significantly associated with elevated PLR, suggesting that PLR could be a new parameter in the current TNM staging system for colorectal cancer prognosis.[19]
In our study, we discovered a statistically significant relationship between PLR and NLR and histological stages of cancer. This is in contrast to a study by Seetohul et al. in 2020, as they saw a non-significant rise in NLR and PLR with an increase in the histological grade of cancer.[20]
Furthermore, NLR decreased from well-differentiated to moderately differentiated and then increased from moderately differentiated to poorly differentiated OSCC. These results are inconclusive and may need further research with a larger sample size. However, poor tumor differentiation was associated with high NLR (P = 0.019) and PLR (P = 0.019) in a study by Zou et al.[21]
We also saw a statistically significant positive correlation between NLR and PLR, which was in concurrence with a finding in the study by Ari and Gunver, who also showed a strong and statistically significant positive correlation between NLR and PLR in thyroid cancer.[22]
Another finding of our study was that the mean MPV levels in the oral cancer group were lower than the control groups but the result was not statistically significant [Table 1]. In a study by Düzlü et al., they also found MPV levels significantly decreased in OSCC as compared to control groups.[16] Some studies have given the opposite results. In a study conducted by Anand et al., mean MPV levels in OSCC patients were higher than controls but there was no statistical association seen between them.[12] Therefore, the role of MPV in the prognosis of OSCC patients remains controversial.
CONCLUSION
We conclude that inflammatory hematological markers such as NLR and PLR act as significant prognostic factors in OSCC. MPV levels were not as significant as NLR and PLR in determining prognosis in OSCC. Furthermore, NLR and PLR are quick and easily measurable parameters that can be used as clinical biological markers to predict the prognosis of cancer.
As the present study was conducted on only 30 patients at one institution, this study has a sample size limitation. Our research, therefore, requires confirmation by larger prospective studies.
Acknowledgment
I am thankful to ICMR-STS for giving me the opportunity to do this research. I thank my seniors, Dr. Sonal Mam, Dr. Rakshita Mam, Dr. Shivangini Mam, and my friend Dr. Sumit.
Ethical approval
The research/study was approved by the Institutional Review Board at Maulana Azad Institute of Dental Sciences, number EC/NEW/INST/2020/1207, dated July 30, 2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Epidemiology of oral cancer in Asia in the past decade-an update (2000-2012) Asian Pac J Cancer Prev. 2013;14:5567-77.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for head and neck cancer in young adults: A pooled analysis in the INHANCE consortium. Int J Epidemiol. 2015;44:169-85.
- [CrossRef] [PubMed] [Google Scholar]
- Squamous cell carcinoma and precursor lesions of the oral cavity: Epidemiology and aetiology. Periodontol. 2000-2011;57:19-37.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular pathogenesis of oral squamous cell carcinoma: Implications for therapy. J Dent Res. 2008;87:14-32.
- [CrossRef] [PubMed] [Google Scholar]
- Combination of platelet count and mean platelet volume (COP-MPV) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patients. Tumour Biol. 2016;37:9323-31.
- [CrossRef] [PubMed] [Google Scholar]
- Elucidating the correlation between treatment with tyrosine kinase inhibitors and mean platelet volume in patients with metastatic renal cell cancer. Oncol Lett. 2014;8:2249-52.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative neutrophil-lymphocyte ratio before platelet-lymphocyte ratio predicts clinical outcomes in patients with cervical cancer treated with initial radical surgery. Int J Gynecol Cancer. 2014;24:1319-25.
- [CrossRef] [PubMed] [Google Scholar]
- Metastasis: New functional implications of platelets and megakaryocytes. Blood. 2016;128:24-31.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio help identify patients with lung cancer but do not differentiate between lung cancer subtypes. Croat Med J. 2016;57:287-92.
- [CrossRef] [PubMed] [Google Scholar]
- Significance of platelet parameters in squamous cell carcinoma of oral cavity-A case-control study. J Cancer Res Ther. 2022;18:1036-41.
- [CrossRef] [PubMed] [Google Scholar]
- The role of blood platelets in tumor angiogenesis. Biochim Biophys Acta. 2011;1815:189-96.
- [CrossRef] [PubMed] [Google Scholar]
- P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci U S. 1998;95:9325-30.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic role of neutrophil-lymphocyte ratio in oral cavity cancers. Niger J Clin Pract. 2018;21:49-53.
- [CrossRef] [PubMed] [Google Scholar]
- Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6:283-7.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil to lymphocyte ratio is an independent prognostic factor in patients with recurrent or metastatic head and neck squamous cell cancer. Mol Clin Oncol. 2015;3:839-42.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic evaluation of platelet to lymphocyte ratio in patients with colorectal cancer. Oncotarget. 2017;8:86287-95.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in head and neck malignancies. Indian J Otolaryngol Head Neck Surg. 2020;72:128-32.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical significance of pre-operative neutrophil lymphocyte ratio and platelet lymphocyte ratio as prognostic factors for patients with colorectal cancer. Oncol Lett. 2016;11:2241-8.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patients with thyroiditis and papillary tumors. J Int Med Res. 2019;47:2077-83.
- [CrossRef] [PubMed] [Google Scholar]







