Translate this page into:
Case of Aeromonas hydrophila necrotizing fasciitis following inconspicuous trauma in an immunocompetent adult host
*Corresponding author: Kavitha Sapru, Department of Anaesthesiology and Critical Care, Aster Medcity, Kochi - 682 027, Kerala, India. kavisapru@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sapru K, Balagopal A, Rajgopal R, Sasidharan A. Case of Aeromonas hydrophila necrotizing fasciitis following inconspicuous trauma in an immunocompetent adult host. Indian J Med Sci 2021;73(2):249-52.
Abstract
Necrotizing fasciitis is a deadly necrotic inflammation of skin, subcutaneous tissues, and muscle bundles. We describe a case of necrotizing fasciitis by Aeromonas hydrophila in an immunocompetent adult host. Although rare, the associated mortality is as high as 60–75%.
Keywords
Aeromonas hydrophila
Debridement
Necrotizing fasciitis
INTRODUCTION
Aeromonas hydrophila is a Gram-negative, motile, oxidase, and catalase-positive, facultative anaerobic bacillus. It is most commonly isolated both from fresh and brackish waters. There are many cases of necrotizing fasciitis by A. hydrophila in immunocompromised patients. However, infections in immunocompetent hosts are rare. We report one such case of morbid necrotizing fasciitis by A. hydrophila in an immunocompetent adult host.
CASE REPORT
A 54 year old female tourist from Europe with no known comorbidities had come to Kerala on a vacation during November 2019. She had been to the backwaters at Alappuzha on November 3 where she had immersed her feet in the water. That day evening, she noticed a small ulcer on her left leg. She could not recall injuring her leg anywhere. On the next day, she developed fever and excruciating pain on her left leg. She was taken to a local hospital where she was symptomatically managed and transferred to our institution.
On presentation to the ED on November 4 at 8 pm, her leg pain had become severe and she noticed redness of her entire leg and progression of the ulcer. On examination, there was also associated edema and erythema up to the calf of her left leg. A few blisters were also noticed. All peripheral pulses were palpable and no necrotic patches were seen. A Doppler ultrasound was done and it showed no evidence of deep venous thrombosis. She was hemodynamically stable on admission and was afebrile. However, over the next few hours, she became tachycardic and hypotension developed. Two sets of blood cultures were taken and she was loaded with intravenous meropenem and clindamycin. She was started on noradrenaline for her hypotension and her blood pressure improved.
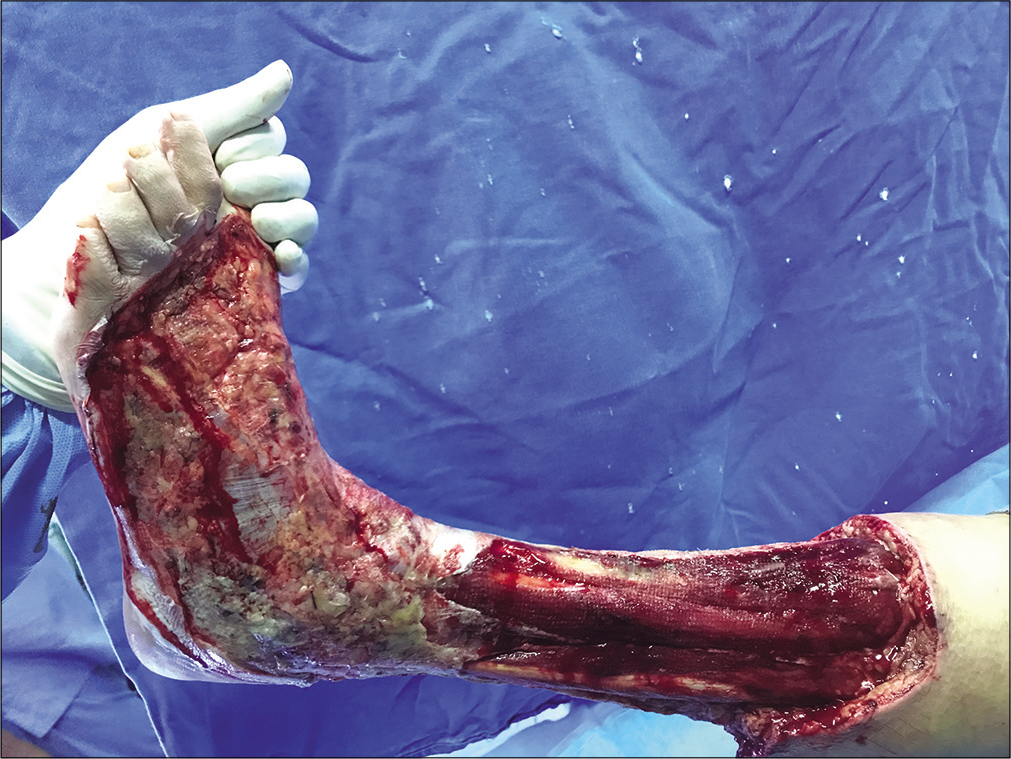
The swelling and erythema of her left leg progressed further with excruciating pain which was managed with intravenous opioids [Figure 1]. There were no other rashes or injuries on her. A diagnosis of necrotizing fasciitis was made and she was taken up for emergency wound debridement. Intraoperatively, hemorrhagic blisters with grayish-black discoloration of skin of the left leg from proximal third to ankle and extending over the dorsum of the foot were noticed [Figure 2]. A sample was taken intraoperatively and sends for bacterial culture. She was electively intubated for the procedure and was extubated postoperatively. She was shifted to MICU for further management [Figure 3].
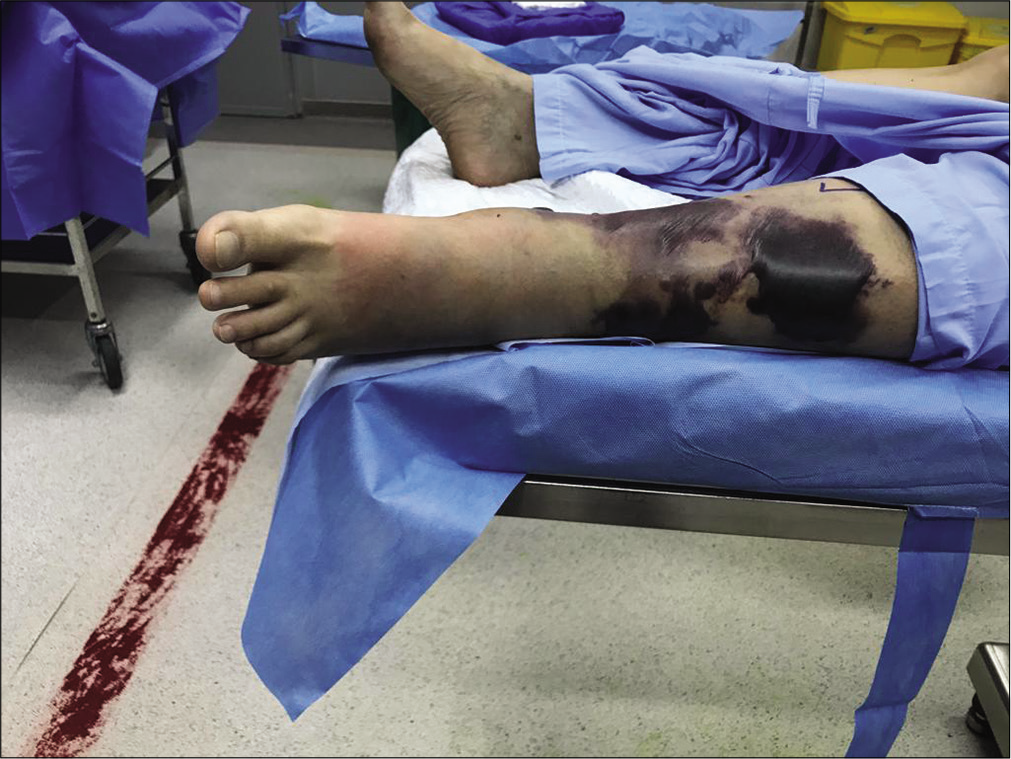
- Pre-operative status of the leg on the day of admission.
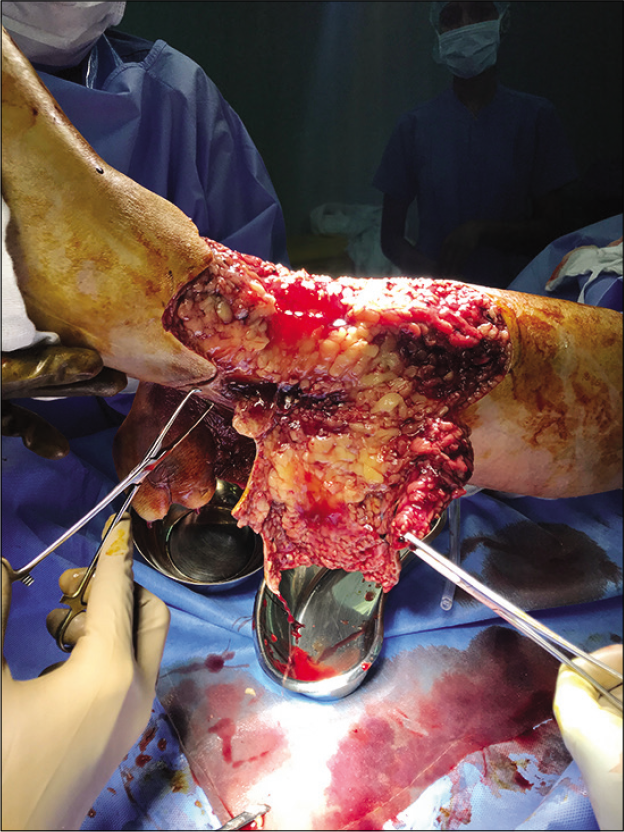
- Intraoperative status of the leg.
- Post-debridement on day 1.
Her blood report showed C-reactive protein of 374 mg/L and procalcitonin of 4.9 ng/ml. Her peripheral smear showed severe panleukopenia with a total count of 140 cells/ml with absolute neutrophil count of 60 cells/ml. Her hemoglobin was 11 g/dl and platelet count was 1.5 lakhs/ml. Her liver functions were also deranged with total bilirubin of 2.1 mg/dl with direct fraction of 2 mg/dl. Her liver enzymes were also elevated – SGOT of 99 U/L and SGPT of 98U/L with an albumin of 2.6 g/dl and INR of 2.1. She was transfused with four fresh frozen plasmas. The rest of her investigations were normal.
A hemato-oncology consultation was sought and leukopenia was thought to be secondary to sepsis and granulocyte macrophage colony-stimulating factor of 300 μg was started. In case she fails to respond, it was decided to go for a bone marrow biopsy. However, her neutrophil counts slowly improved and biopsy was deferred. A gastromedicine consultation was taken in view of the deranged liver function test. The acute liver injury was presumed to be secondary to the sepsis and she was started on N-acetyl cysteine infusion of 10 g/day.
Her vasopressor support of noradrenaline was tapered off over the next 2 days and was stopped on November 7. She was also given albumin infusion for 3 days in view of hypoalbuminemia and intravascular depletion along with vasopressor requirement. She was continued on meropenem and clindamycin along with doxycycline to cover for tropical infections including scrub typhus.
On November 8, her bacterial cultures sent on November 4 during the initial debridement grew heavy growth of A. hydrophila, which was sensitive to ciprofloxacin, piperacillin-tazobactam, and levofloxacin. Meropenem and doxycycline were stopped and intravenous clindamycin was added. She was also started on fluconazole for oropharyngeal candidiasis which was diagnosed during an endoscopy for the evaluation of her persistent dysphagia and heartburn which was not responding to supportive therapy.
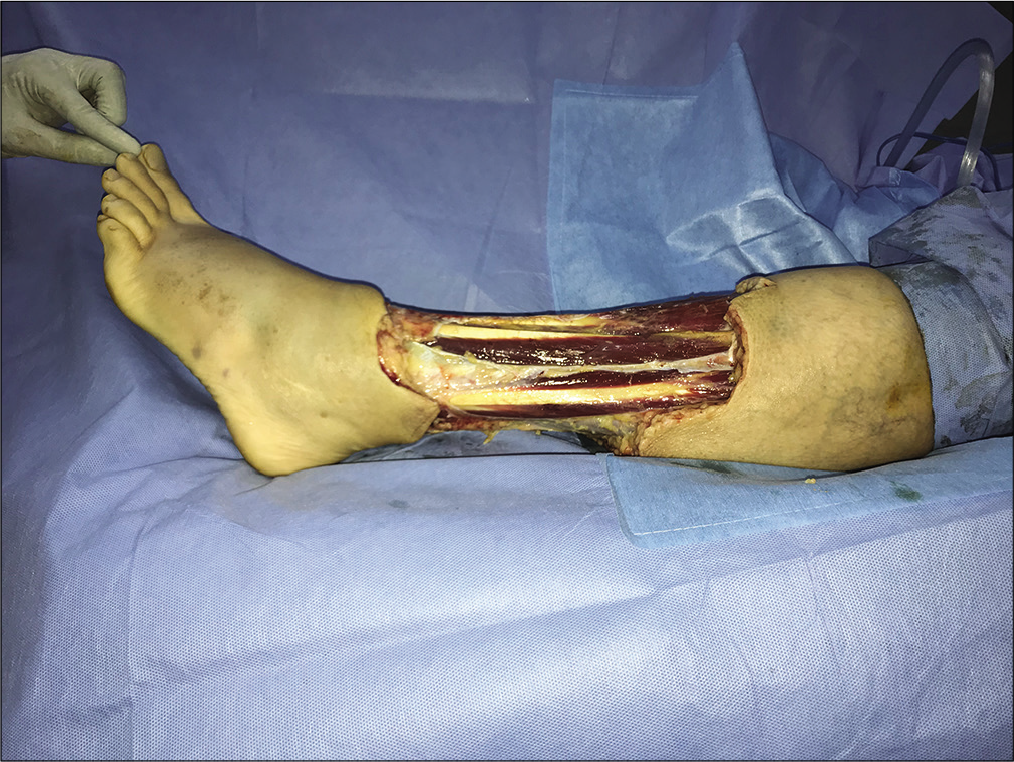
During dressing change on November 8, there was progression of erythema over the left dorsum and calf [Figure 4]. She was taken up immediately for debridement. Intraoperatively, grayish discoloration of skin over posterior aspect of upper one-third of leg and dorsum of foot was noticed. There was also erythema over popliteal fossa with small blisters and extension of erythema over the lateral aspect of the left thigh. The wound was thoroughly debrided and negative pressure wound dressing was done. A MALDITOF sent on the same day also detected A. hydrophila [Figure 5]. The repeat tissue culture send on November 8 showed only scanty growth of A. hydrophila. However, her blood cultures at no point in time grew A. hydrophila.
- Third day post-operative showing progression of erythema on the dorsum.
- Post-debridement of the dorsum of foot.
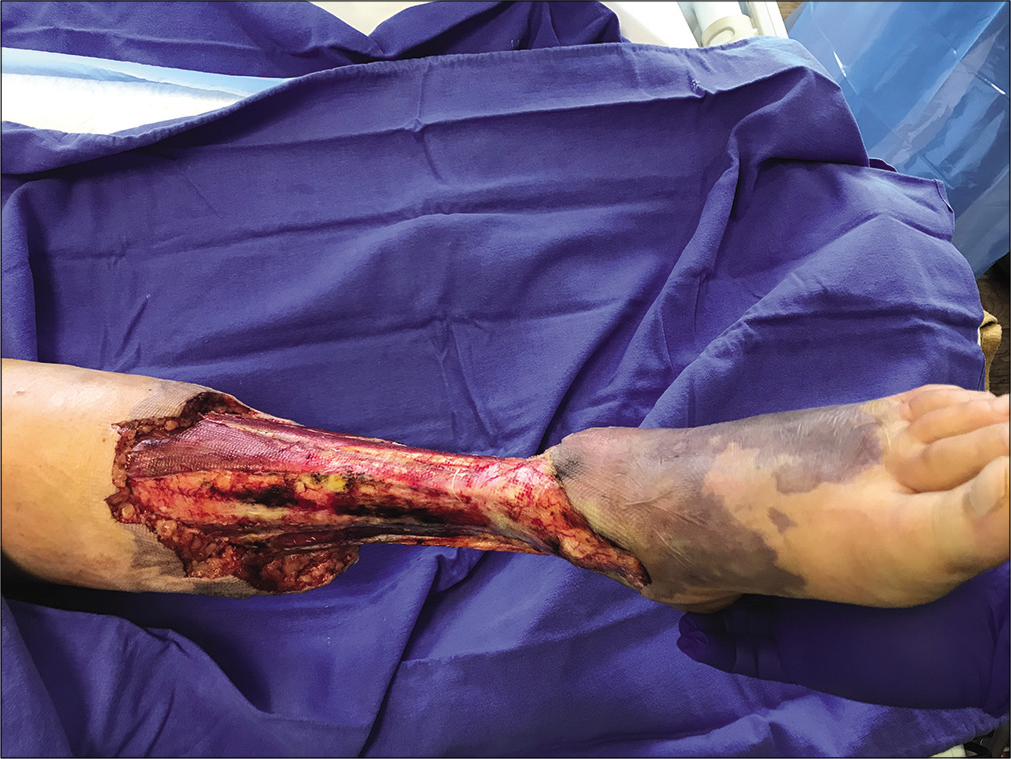
Over the course of next few days, her clinical condition and laboratory parameters showed significant improvement. The total white blood cell count improved to 1700 cells/ml and her liver functions improved with SGOT and SGPT declining to 57 U/L and 249 U/L, respectively. She underwent another wound debridement on November 14. Intraoperatively, there was a soft-tissue defect circumferentially on the left leg and dorsum of the foot with healthy wound bed, exposed musculotendinous unit of the left leg and foot. She was shifted to the ward on November 16 with intravenous ciprofloxacin and fluconazole. She had an uneventful course in the ward and was discharged on November 22 almost 3 weeks after admission with a salvaged limb [Figure 6]. She was advised to continue oral ciprofloxacin and fluconazole and consult a hospital at her home country for further management and rehabilitation. In the course of next 3 months, she underwent a flap surgery and two skin grafting procedures. She had an uneventful recovery and is currently able to walk and even drive the car.
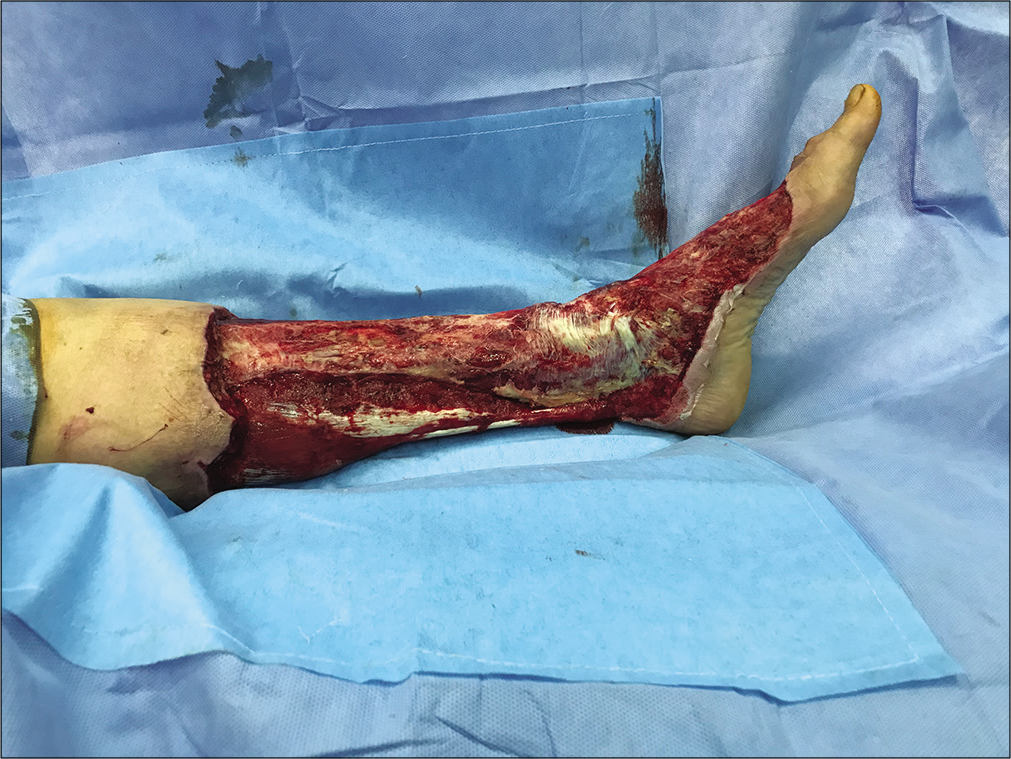
- The day before discharge post-multiple debridements.
DISCUSSION
A. hydrophila is a Gram-negative, motile, oxidase, and catalase-positive anaerobic bacillus that is found in both fresh and brackish waters. Aeromonas is most commonly associated with gastrointestinal illness, mainly diarrhea and they are usually self-limiting.[1] The skin and soft-tissue infections by Aeromonas range from cellulitis to life-threatening necrotizing fasciitis and myonecrosis. The various modes of transmission include ingestion of contaminated food, water contaminated with soil/feces, and exposure of wounds to the water bodies containing the bacteria.[2]
Necrotizing fasciitis by Aeromonas are rare but the associated mortality is as high as 60–75% in immunocompromised hosts. However, there has been an substantial increase in cases of necrotizing fasciitis in the recent years.[3,4] Majority of cases of fulminant necrotizing fasciitis occur following a history of trauma, mostly sustained in an aquatic environment. However, fatal cases of Aeromonas myonecrosis and gas gangrene without any antecedent trauma or associated comorbidities such as liver disease, malignancy, or immunosuppression are rarely reported.
The most common presentation is non-bacteremic cellulitis and the usual predisposing factors are previous surgeries or local trauma in an aqueous environment. The proposed mechanisms are that the bacteria invade through the trauma area which causes primary skin and soft-tissue infection followed by secondary sepsis. The alternate mechanism proposed is sepsis induced by bacteria that are followed by secondary metastatic lesions of skin and soft tissue.[4] Local findings of Aeromonas fasciitis include skin tenderness, erythema, edema, and warm skin. This often gradually progresses to blisters and bullae formation due to the ischemia-induced necrosis. Pathognomonic of early necrotizing fasciitis is the lack of primary muscular involvement. However, once muscular involvement occurs, it is a sign of advanced necrotizing fasciitis and is often associated with grave prognosis and poor survival.
Virulence of Aeromonas depends on several factors and is not completely understood. They attach to the host and enter into the cells through the production of flagella, pili, and adhesins. Inside the host cell, they produce extracellular hydrolytic enzymes such as enterotoxins, proteases, phospholipases, and hemolysins which cause damage to host cells leading to cell death. On the blood agar, they produce distinctive colonies with or without hemolysis. The colonies are then screened by carrying out oxidase test and identified using biochemical methods/kits.
There has been a case report of infection with A. hydrophila of wounds and blood stream of a young immunocompetent patient.[5] It was initially considered to be a monomicrobial necrotizing fasciitis, genomic analysis indicated mixed infection due to four different strains (NF1, NF2, NF3, and NF4) of A. hydrophila. Among the strains, NF2, NF3, and NF4 were characterized as a clonal group with NF2 presenting as the dominant colony morphotype. Genomic analysis of NF1 strain demonstrated a phylogenetically distant relationship to the other three strains.[6] It was speculated that the presence of multiple strains of A. hydrophila influenced disease progression and outcome significantly than if the individual strain had been involved alone. It was thought that the toxin produced by one strain would have had an influential role in the pathogenesis of the other strains during infection.[7]
The management of Aeromonas necrotizing fasciitis is by prompt clinical diagnosis followed by aggressive debridement. The early signs of an infection by Aeromonas are rapid onset of cellulitis within 72 h after an injury which is characterized by pain, swelling, hemorrhagic bullae, subcutaneous bleeding, purpura, necrosis, and gangrene.[8] Aeromonas are susceptible to a wide range of antibiotics such as chloramphenicol, tetracycline, trimethoprim-sulfamethoxazole, aminoglycosides, broad- spectrum cephalosporins, and carbapenems.[9] They, however, exhibit universal resistance to penicillins, ampicillin, carbenicillin, and cefazoline.[10]
In our case, the patient was started on broad-spectrum antibiotics and taken up for emergency debridement in view of the rapidly evolving necrotizing fasciitis. On the detection of Aeromonas in cultures, the antibiotic was later deescalated to ciprofloxacin based on the bacterial sensitivity. The same organism was confirmed by MALDITOF. The whole clinical scenario was complicated with neutropenia and deranged liver functions which improved with resolution of sepsis. Prompt initiation of antibiotics and emergency debridement followed by further debridement and negative pressure wound dressings helped in the clinical improvement of the patient and salvage of the limb.
CONCLUSION
Necrotising fasciitis by Aeromonas hydrophilia is rare, however, it carries a high risk of morbidity and mortality especially if diagnosis is delayed and not aggressively managed. The potential of this organism to cause severe skin and soft tissue infection both in immunocompromised as well as in normal hosts, especially following trauma should be borne in the minds of the clinicians. Management of invasive necrotising fasciitis by Aeromonas include prompt surgical debridement and broad spectrum antibiotics with documented sensitivity.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Aeromonas species in septicemia: Laboratory characteristics and clinical observations. Clin Infect Dis. 1994;19:77-83.
- [CrossRef] [PubMed] [Google Scholar]
- Necrotizing fasciitis caused by inconspicuous infection of Aeromonas hydrophila in an immunocompromised host. J Surg Case Rep. 2010;2010:2.
- [CrossRef] [PubMed] [Google Scholar]
- Necrotizing fasciitis caused by Aeromonas hydrophila. Heart Lung. 2000;29:306-8.
- [CrossRef] [PubMed] [Google Scholar]
- Aeromonas hydrophila infections of skin and soft tissue: Report of 11 cases and review. Clin Infect Dis. 1993;16:69-74.
- [CrossRef] [PubMed] [Google Scholar]
- Functional genomic characterization of virulence factors from necrotizing fasciitis-causing strains of Aeromonas hydrophila. Appl Environ Microbiol. 2014;80:4162-83.
- [CrossRef] [PubMed] [Google Scholar]
- Cross-talk among flesh-eating Aeromonas hydrophila strains in mixed infection leading to necrotizing fasciitis. Proc Natl Acad Sci USA. 2016;113:722-7.
- [CrossRef] [PubMed] [Google Scholar]
- Presentation and outcomes of necrotizing soft tissue infections. Int J Gen Med. 2017;10:215-20.
- [CrossRef] [PubMed] [Google Scholar]
- Necrotizing fasciitis caused by Aeromonas caviae. Avicenna J Med. 2012;2:94-6.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro susceptibilities of Aeromonas genomic species to 69 antimicrobial agents. Syst Appl Microbiol. 1999;22:662-9.
- [CrossRef] [Google Scholar]
- Aeromonas hydrophila infection: Clinical aspects and therapeutic options. Rev Med Microbiol. 2002;13:151-62.
- [CrossRef] [Google Scholar]






