Translate this page into:
Depression and Quality of Life of Parkinson’s disease patients – A hospital-based cross-sectional study
*Corresponding author: Maanasa Rajagopalan, Department of Epidemiology, The Tamil Nadu Dr. MGR Medical University, Chennai, Tamil Nadu, India. maanasarajagopal.95@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Rajagopalan M, Ranganathan LN, Valarmathi S, Govindarajulu S, Seshayyan S. Depression and Quality of Life of Parkinson’s disease patients – A hospital-based cross-sectional study. Indian J Med Sci 2022;74:57-61.
Abstract
Objective:
The objective of this study was to assess the levels of depression among patients with Parkinson’s Disease (PD), the factors influencing their levels, and determine their influence on their Quality of Life (QoL).
Materials and Methods:
The study was conducted at the movement disorders outpatient department, Rajiv Gandhi Government General hospital, where 50 patients with diagnosed PD were enrolled. They were interviewed for their levels of depression and QoL using respective standardized depression and QoL assessment questionnaires.
Results:
Patients with PD were mostly above the age of 50 and were male. Their levels of depression ranged between normal to mild and borderline depression and their QoL scores were also moderate. Among their QoL domains, the emotional well-being, and cognition of patients were found to be more affected when compared to other domains.
Conclusion:
The prevalence and severity of depression among patients with PD were mild, but they play an important role in determining their QoL, especially the emotional well-being and cognitive performance of patients.
Keywords
Parkinson’s disease
Depression
Quality of life
HIGHLIGHTS
The study focuses on the level of depression of patients with Parkinson’s disease and its influence on their quality of life. The depression levels of most patients were ranging from mild to moderate, yet, they are found to have a significant negative impact on their quality of life. Thus, their emotional well-being requires care along with their pathological symptoms for better results.
INTRODUCTION
Chronic neurodegenerative diseases are increasingly recognized as the most common causative factor determining disability and death worldwide. The diseases include Multiple Sclerosis, Alzheimer’s disease, Parkinson’s disease (PD), Huntington’s disease, and much more, of which Alzheimer’s disease is the most common one followed by Parkinsonism.
PD includes a group of disorders that affect the motor response and mobility of individuals and the disease is increasing gradually worldwide. The disease is clinically categorized as a movement disorder with prominent motor symptoms which are the result of gradual degeneration of nerve cells, in the portion of the midbrain that controls body movements.[1] Certain changes occur in the brain of patients which include – the presence of Lewy bodies and Alpha-synuclein[2] which are important microscopic markers that hold an important clue to the cause of the disease.
The overall prevalence of PD as per lancet’s study 2016 is around 6.1 million which was around 2.5 million in 1990, thus indicating a 21% increase.[3] Thus, PD prevalence has doubled over the past generation, thus becoming a major cause of disability due to a motor problem. The risk factors of the disease include older age, especially above 60 years with the heredity of having a close relative with PD and exposure to various toxins such as herbicides and pesticides. Men are found to be at a greater risk of developing the disease.[4]
Recent epidemiological studies suggest that various comorbidities are associated with the development of PD, of which some may increase the risk of disease and precede the onset of motor symptoms.[5] Of all the comorbidities, depression, anxiety, cognitive impairment, and sleep disturbances are common neuropsychiatric manifestations of PD.[6] Depressive disturbances are common in patients with PD and influence many other clinical aspects of the disease.
An association between PD and depression has been established in both community and hospital-based studies. The prevalence of moderate-to-mild depression in PD is 24.1– 45.5% in community-based studies to 71% in hospital-based studies, especially in women. A recent systematic review indicates that depression is present in 52% of PD patients.[7]
Thus, a study on the prevalence and severity of depression among patients with PD would be helpful to understand the risk. Furthermore, correlating the depression levels with the quality of life (QoL) of patients will help us know their relationship and this would facilitate planning for the early detection and appropriate treatment for patients which would help in improving their QoL, as it is more essential to be good enough, for the success of treatment and to curb the process of progression.
Objectives
The objective of this study was to assess the levels of depression among patients with PD, the factors influencing their levels and determine their influence on the QoL of patients.
MATERIALS AND METHODS
The study was conducted at the movement disorders outpatient department, Rajiv Gandhi Government General hospital Chennai, where 50 patients with diagnosed PD were enrolled. They were interviewed using Beck’s Depression Inventory (BDI) questionnaire to assess their levels of depression and their QoL was then assessed using PD Questionnaire (PDQ-39). Their depression levels were, then, assessed and correlated with the QoL of patients to understand the nature and influence of their relationship.
All patients were given an informed consent form and patient information sheet regarding the purpose and mode of study before the commencement of the study and only those who gave their consent were taken up for the study. This study was approved by the Local Institutional Ethics Committee (IRB No. 39062019).
Score interpretation and analysis plan
Depression: BDI questionnaire
The depression levels can be assessed and staged using the BDI questionnaire which includes six levels based on scores (Out of 100), where 1–10 is considered normal, 11–16 is mild mood disturbance, 17–20 is borderline depression, and 21– 30 is considered as moderate depression. A score above 31 is considered severe depression.
QoL: PDQ-39 questionnaire
PDQ-39 scale has eight domains of assessment, namely, mobility, activities of daily living, emotional well-being, stigma, social support, cognition, communication, and bodily discomfort. The scores are made on a five-point Likert scale from 0 (“Never”) to 4 (“Always”) which are converted to a 0–100 scale for interpretation. The resulting scores mean in reverse, where a score of 0 indicates good QoL, while a score of 100 indicates poor QoL.
The resulting scores of depression and QoL were analyzed and compared using a parametric t-test, Chi-square test, and Pearson’s correlation coefficient. Modeling and prediction regarding depression were evaluated using multiple linear regression techniques.
Multiple regression model
The multiple regression model used in our study was to predict the values of depression, the dependent variable, using other variables such as the demography of patients and their QoL scores as these values are known to cause a significant difference to the other. It will help to determine the overall fit of the model and the relative combination of each predictor to the total variance as our dataset met all assumptions required.
The R-value representing the multiple correlation coefficient measures the quality of prediction and the R2 value explains the proportion of variance. The F value explains the test measured to see, whether the overall regression model is a good fit for the data and the model coefficient values (B, Beta) indicate how much the depression scores (dependent variable) vary with independent variables.
RESULTS
PD patients reported for the study were mostly above the age of 60 (68%) and were mostly males (64%). Regarding females, 56% of women were above the age of 50 years [Figure 1]. The age range was 29–78 and their mean age was 55.4.
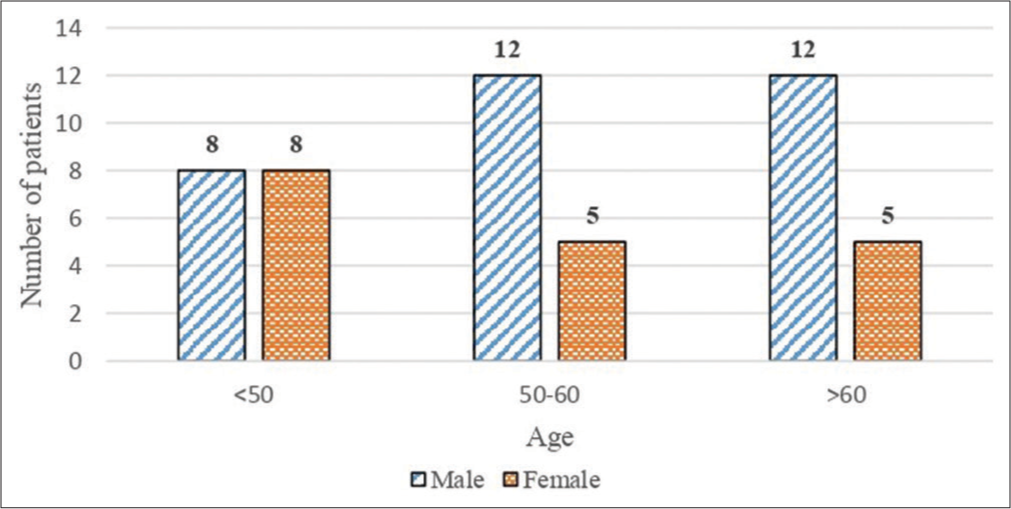
- Demographic distribution
Regarding their levels of depression, 32% of patients with PD had no depression, while 68% had mild mood disturbance or borderline depression and their mean score was 8.9 [Table 1]. Patients with no depression were mostly <50 years of age and most patients with mild depression (Stage I) were above 60 years, showing a direct correlation [Figure 2] between depression levels and the age of patients (r = 0.353).
| Depression levels (Score) | PD | ||||
|---|---|---|---|---|---|
| Frequency (%) | CI | ||||
| <50 | 50–60 | >60 | Total | ||
| No/Normal (<10) | 14 (28) | 2 (4) | - | 16 (32) | 0.54–0.79 |
| Mild mood disturbance (11–16) | 13 (26) | 4 (8) | - | 17 (34) | 0.17–0.41 |
| Borderline clinical depression (17–20) | 7 (14) | 8 (16) | 2 (4) | 17 (34) | 0.01–0.13 |
| Total | 50 | ||||
| Mean Score (SD) | 8.9 (4.017) | ||||
PD: Parkinson’s disease
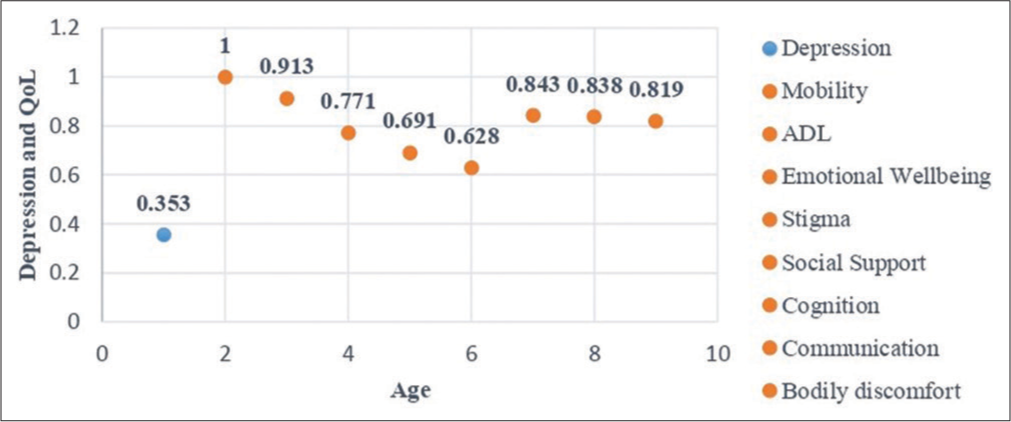
- Age - depression - QoL correlation levels
On assessing the QoL of patients, their average mean score was 32.39 which indicates moderate QoL [Table 2]. The age of patients positively correlated with their QoL (r = 0.6–1.0) indicating an increase in age would affect their QoL in an existing pattern.
| QoL Domains – Depression | Correlation (r-value) | P-value |
|---|---|---|
| Mobility | 0.543 | <0.05 |
| ADL | 0.442 | <0.05 |
| Emotional well-being | 0.703 | <0.05 |
| Stigma | 0.593 | <0.05 |
| Social support | 0.504 | <0.05 |
| Cognition | 0.607 | <0.05 |
| Communication | 0.536 | <0.05 |
| Bodily discomfort | 0.440 | <0.05 |
Depression and QoL
The levels of depression are usually reported to have a higher influence on the QoL of patients with any chronic neurodegenerative illnesses, especially PD. Thus, in our study too, we aimed to determine the influence of depression on the QoL of patients with PD.
Our study evaluated the depression levels of patients, and most of them showed mild or moderate signs of depression [Table 1]. This when correlated with their QoL revealed that depression levels correlated positively with (r = 0.55), where patients with no depression showed moderate or better QoL, while patients with depression showed its influence in their life indicating low QoL. Regarding the domains, depression seems to affect the emotional well-being (r = 0.703) and cognition (r = 0.607) of patients more when compared to other domains, disturbing their QoL [Table 2].
Factors influencing depression levels
The depression levels assessed among PD patients showed their levels and their relationship with their demographic factors (age and gender) and their QoL domains. The values were, then, standardized and correlated using a multiple regression model to predict their influence on the depression level of patients with time.
The results showed that among patients with PD, the variables that significantly predicted (P < 0.05) or had an influence on depression levels were three QoL domains – Bodily discomfort, Emotional well-being, and Cognition [Table 3]. An R-value of 0.793 indicates a good level of prediction. Age and gender values were insignificant.
F (3, 47) = 26.48, P < 0.05 and R2 = 0.63
| Variables | Unstandardized coefficient | Standardized coefficient | T | Significance | CI | |
|---|---|---|---|---|---|---|
| B | Standard error | Beta | ||||
| Emotional well-being | 0.851 | 0.158 | 0.851 | 5.380 | <0.05 | 0.533–1.170 |
| Cognition | 0.619 | 0.187 | 0.619 | 3.305 | <0.05 | 0.242–0.995 |
| Bodily discomfort | −0.785 | 0.196 | −0.785 | −4.001 | <0.05 | −1.180–0.390 |
Dependent variable: Depression
Thus, for each one-point increase in their emotional well-being and cognition scores, there will be an increase of 0.85 and 0.62 in their depression scores. While the bodily discomfort score has a negative effect, where an increase of one in their score will result in a decrease of 0.785 (B = −0.785) in depression scores and vice versa.
Factors influencing QoL measures
The QoL domains assessed among PD patients showed their levels and their relationship with age, gender, and depression levels. The values were, then, standardized and correlated using multiple regression models to predict their influence over patients’ QoL domains with time.
The results showed that age and depression levels of PD patients had a significant influence and gender had little to no significance over their QoL. Among the QoL domains, age and depression levels determined all domains at an average R-value of 0.8, except the bodily discomfort domain which had an average prediction of 0.5 [Table 4].
| Dependent variables (QoL Domains) | Independent Variables | R value | Unstandardized coefficient | Standardized coefficient | T | CI | |
|---|---|---|---|---|---|---|---|
| B | Standard error | Beta | |||||
| Mobility | Age | 0.93 | 0.292 | 0.016 | 0.292 | 18.434 | 0.26–0.324 |
| Depression | 0.93 | 1.751 | 0.096 | 1.751 | 18.191 | 1.55–1.94 | |
| ADL | Age | 0.925 | 0.164 | 0.01 | 0.164 | 17.067 | 0.145–0.184 |
| Depression | 0.918 | 0.979 | 0.061 | 0.979 | 16.167 | 0.857–1.101 | |
| Emotional Well-being | Age | 0.897 | 0.128 | 0.009 | 0.128 | 14.186 | 0.11–0.146 |
| Depression | 0.943 | 0.807 | 0.041 | 0.807 | 19.794 | 0.72–0.88 | |
| Stigma | Age | 0.92 | 0.102 | 0.006 | 0.102 | 16.476 | 0.09–0.115 |
| Depression | 0.93 | 0.625 | 0.034 | 0.625 | 18.415 | 0.55–0.69 | |
| Social support | Age | 0.731 | 0.04 | 0.005 | 0.04 | 7.491 | 0.03–0.05 |
| Depression | 0.804 | 0.267 | 0.028 | 0.267 | 9.46 | 0.21–0.32 | |
| Cognition | Age | 0.904 | 0.086 | 0.006 | 0.086 | 14.832 | 0.075–0.098 |
| Depression | 0.925 | 0.53 | 0.031 | 0.53 | 16.98 | 0.46–0.59 | |
| Communication | Age | 0.882 | 0.068 | 0.005 | 0.068 | 13.106 | 0.058–0.078 |
| Depression | 0.908 | 0.421 | 0.028 | 0.421 | 15.184 | 0.36–0.48 | |
| Bodily discomfort | Age | 0.577 | 0.083 | 0.017 | 0.083 | 4.943 | 0.049–0.117 |
| Depression | 0.596 | 0.517 | 0.1 | 0.517 | 5.193 | 0.32–0.72 | |
Level of significance<0.05
On average, an R-value of 0.86 indicates a good level of prediction. Gender values were insignificant.
Thus, for each point increase in depression score, there is an average increase of one point in mobility, ADL, and emotional well-being score and a 0.5 increase in other domains of QoL of PD patients. However, age and QoL domain prediction levels were not much of difference.
DISCUSSION
Our study, thus, shows that less number of patients with PD (28%) suffer from depression. This contradicts various other findings like the cross-sectional study by Arun et al. 2011, where 54% of their patients with PD were found to have stage II depression.[8] Our study also showed that the QoL of PD patients was moderate and is interpreted to be due to their depression levels which are also quite low.
However, this is contradictory to a study by Lucas-Carrasco et al. 2011 and a review by Batista and Pereira. 2015 on the QoL of patients with chronic neurodegenerative diseases including PD, where they stated that, among all diseases, patients with PD, and multiple sclerosis presented lower levels of QoL.[9,10]
On assessing the level of influence of depression on QoL of PD patients, depression showed significant influence over three main domains of QoL, namely, mobility, activities of daily living, and emotional well-being of PD patients, while age and gender levels were insignificant. This is similar to a study by Kuhlman et al. 2019, where non-motor symptoms accounted for a 48% variance in QoL domain scores and were significantly correlated.[11]
Thus, our study supports the previous findings concluding that depression levels of patients have a significant influence over their QoL, though the levels may vary with other factors.
CONCLUSION
Therefore, the measurement of the prevalence and severity of levels of depression in patients with PD explicitly explains that they have mild-to-moderate levels of depression. This appears to have a powerful enough impact on emotional well-being and cognitive behavior to exacerbate their illness and ultimately harm their QoL. This study, thus, recommends patients be screened and treated for depression through adequate therapy with particular emphasis on their mental well-being, to minimize the progression of the disease and its effects.
Limitations
Our study is comparatively small with 50 participants and though our findings are significant, some results may differ if more participants were added
The stage of disease of patients (UPDRS scoring) and their amount of disability, which could be potential confounders, could not be included for assessment and correlation. Their relationship needs to be explored as it may be significant.
Ethical approval
IEC approval was obtained from the Madras Medical College Institutional Ethics committee (No.39062019), where the study was conducted.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Parkinson's disease and Parkinsonism: Neuropathology. Cold Spring Harb Perspect Med. 2012;2:a009258.
- [CrossRef] [PubMed] [Google Scholar]
- Global, regional, and national burden of Parkinson's disease, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939-53.
- [CrossRef] [Google Scholar]
- Risk factors for Parkinson's disease. Neurology. 1993;43:1693-7.
- [CrossRef] [PubMed] [Google Scholar]
- Biological and clinical implications of comorbidities in Parkinson's disease. Front Aging Neurosci. 2017;9:394.
- [CrossRef] [PubMed] [Google Scholar]
- Depression and Parkinson's disease: Current knowledge. Curr Neurol Neurosci Rep. 2013;13:409.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of Parkinson's Disease. Available from: http://viartis.net/parkinsons.disease/prevalence.htm [Last accessed on 2020 Aug 21]
- [Google Scholar]
- Relationship of depression, disability, and quality of life in Parkinson's disease: A hospital-based case-control study. Neurol India. 2011;59:185-9.
- [CrossRef] [PubMed] [Google Scholar]
- Using the WHOQOL-DIS to measure quality of life in persons with physical disabilities caused by neurodegenerative disorders. Neurodegener Dis. 2011;8:178-86.
- [CrossRef] [PubMed] [Google Scholar]
- Quality of life in patients with neurodegenerative diseases. J Neurol Neurosci. 2016;7:1-7. Available from: https://www.jneuro.com/abstract/quality-of-life-in-patients-with-neurodegenerative-diseases-8682.html [Last accessed on 2020 Aug 21]
- [CrossRef] [Google Scholar]
- Predictors of health-related quality of life in Parkinson's disease. Parkinsonism Relat Disord. 2019;65:86-90.
- [CrossRef] [PubMed] [Google Scholar]






