Translate this page into:
Flexor hallucis longus augmentation for Achilles tendon – is pre-operative evaluation of flexor hallucis longus by MRI required?
*Corresponding author: Dr Rajesh Botchu, Department of Musculoskeletal Radiology, Royal Orthopedic Hospital, Bristol Road South, Birmingham, United Kingdom. drbrajesh@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Saad A, McLoughlin E, Hanif U, Iqbal A, James S, Botchu R. Flexor hallucis longus augmentation for Achilles tendon – is pre-operative evaluation of flexor hallucis longus by MRI required? Indian J Med Sci 2021;73(2):197-202.
Abstract
Objectives:
Flexor hallucis longus (FHL) tendon transfer is the method of choice in reconstructing chronic neglected Achilles tendon rupture. We performed a retrospective study to assess the incidence and degree of fatty degeneration of FHL.
Material and Methods:
Two hundred and twenty-five consecutive MR of ankles were reviewed retrospectively and assessed for fatty atrophy based on Goutallier classification.
Results:
About 42.7% had Grade 1, 8.4% had Grade 2, 3.1% had Grade 3, and 1.8% had Grade 4 fatty atrophy of FHL. Other lesions identified included posterior ankle impingement, tenosynovitis, loose bodies, and giant cell tumors of the tendon sheath.
Conclusion:
We suggest pre-operative radiological assessment of the FHL to establish that the FHL muscle and tendon are normal and intact and suitable for transfer surgery. We also discuss the spectrum of pathologies affecting FHL.
Keywords
Flexor
Hallucis
Longus
Tendon
INTRODUCTION
The flexor hallucis longus (FHL) is one of the three deep muscles located in the posterior compartment of the leg and is the second strongest muscle in the human body. It is sometimes referred to as the “Achilles tendon” of the foot in dancers, due to its anatomical nature, closely approximating the toughness, length and contractile force of the Achilles tendon (AT).[1] Therefore, it has become increasingly common to harvest and utilize FHL in the treatment of neglected (chronic) AT rupture (ATR).[2,3]
Transfer of the FHL to augment the AT is an established surgical procedure and has excellent results with good pain relief and functional outcome.[2] The main purpose of this procedure is to restore the original length and strength of the AT. For it to be successful, the FHL muscle body must be relatively normal.[4]
Studies on the different pathologies of the FHL tendon and their potential effects on the FHL tendon transfer procedure for chronic ATR are sparse. Our aim, in this paper, is to review and highlight the spectrum of pathologies that involve the FHL, with the emphasis on the awareness and diagnosis of these lesions before the management of ATR, to decrease morbidity and potential failure of the FHL transfer procedure.
MATERIAL AND METHODS
We performed a retrospective review of (225) consecutive MR images of ankle performed at our institution during 2017 for ankle pain and identified the extent of fatty infiltration of the FHL muscle using a Goutallier classification [Table 1]. We also looked at a spectrum of other pathologies that were found to affect the FHL. Ethical approval from our institution was obtained to undertake this study.
| Grade | Amount of fat in muscle |
|---|---|
| Grade 0 | Normal muscle |
| Grade 1 | Some fatty streaks |
| Grade 2 | Less than 50% fatty muscle atrophy |
| Grade 3 | 50% fatty muscle atrophy |
| Grade 4 | Greater than 50% fatty muscle atrophy |
RESULTS
The average age of our cohort was 44 years (10–82 years) with a female predominance (130 female and 95 males). About 44% of the cohort had normal FHL, 42.7% demonstrated grade 1 fatty atrophy and further small proportion of patients with Grade 2 (8.4%), Grade 3 (3.1%), and Grade 4 (1.8%) fatty atrophy [Table 2].
| Goutallier | Number | Percentage |
|---|---|---|
| Grade 0 | 99 | 44 |
| Grade 1 | 96 | 42.7 |
| Grade 2 | 19 | 8.4 |
| Grade 3 | 7 | 3.1 |
| Grade 4 | 4 | 1.8 |
Other lesions identified included posterior ankle impingement, tenosynovitis, loose bodies, and giant cell tumors of the tendon sheath.
DISCUSSION
The FHL is found deep in the posterior compartment of the leg and is accompanied by two further muscles; the tibialis posterior (TP) and flexor digitorum longus (FDL). It originates from the posterior aspect of the lower part of the fibula and the interosseous membrane. The FHL courses along with FDL and TP through the tarsal tunnel on the medial side of the ankle under the flexor retinaculum [Figures 1 and 2]. It then courses through a groove found between the medial and lateral tubercles of the posterior portion of the talus. The FHL tendon begins just above the level of the medial malleolus and is lined by a synovial sheath. Several studies have established a connection between the ankle joint and the FHL.[5,6].In the midfoot, at the level of the navicular bone, the FHL tendon is crossed over by the FDL. This area is known as the master knot of Henry. Distal to the knot of Henry there are connections, sometimes multiple, between the two tendons. This area is an important surgical landmark and is used for harvesting the tendon grafts for the treatment of ATR.[7].The tendon ends its journey by inserting on the plantar surface of the base of the distal phalanx of the great toe. The primary function of the FHL is to flex the great toe, but also aids the foot in plantar flexion.

- Diagrammatic representation of the sagittal image of the ankle demonstrating the flexor hallucis longus.
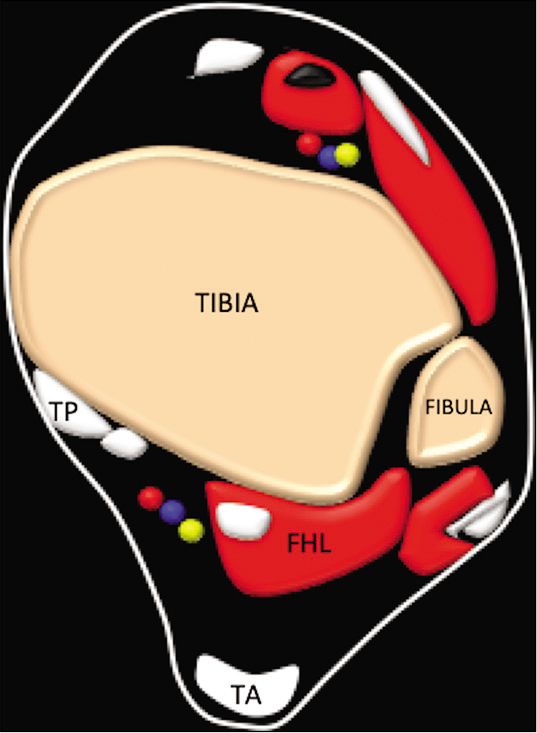
- Diagrammatic representation of the axial image of the ankle demonstrating the flexor hallucis longus, tibialis posterior (TP) tendon Achilles.
Fatty infiltration within skeletal muscle is a recognized normal phenomenon that occurs with ageing and is thought to impair the muscle force production with an effect on the mobility and metabolic status in the elderly.[8] Skeletal muscle fatty infiltration (SMFI) can also be seen in younger individuals and is understood to be related to various risk factors including severe muscle injury and/or chronic musculotendinous tears, immobilization, and/or chronic disuse.[9]
SMFI has been used as a prognostic factor to guide the management of different surgical procedures.[10-12] Studies have shown that skeletal muscle lipid content influences muscle strength and has a strong correlation with the maximal muscle tension.[4] Several papers have demonstrated that intramuscular lipid may influence muscle strength. The consensus is that any increase in intermuscular fat decreases the insulin sensitivity, which, in turn, impairs normal protein synthesis, resulting in a direct effect on muscle, mass, and strength.[13-15]
MR has been used as the primary imaging modality to assess for SMFI. It is characterized by the manifestation of fat signal intensity within the muscle belly on both T1-weighted and T2-weighted sequences.[16] The Goutallier classification is used in quantifying the amount of fatty infiltration in the rotator cuff muscles using MRI. It is based mainly on the percentage of fatty atrophy of the involved muscle and has five grades [Table 1].[17,18]
With our findings, we can hypothesize that fatty infiltration is a common finding in the FHL muscle. We suggest cross-sectional imaging to assess the muscle belly before tendon transfer surgery for chronic ATR [Figure 3]. Ideally, FHL with Goutallier classification Grade 0 would be preferred for FHL augmentation for AT but if not the Grades 1 and 2 could also be used. FHL with 50% or more fatty atrophy (Goutallier classification – Grades 3 and 4) are likely to be associated with relatively poor results [Figure 4]. A prospective study to analyze this would be required.
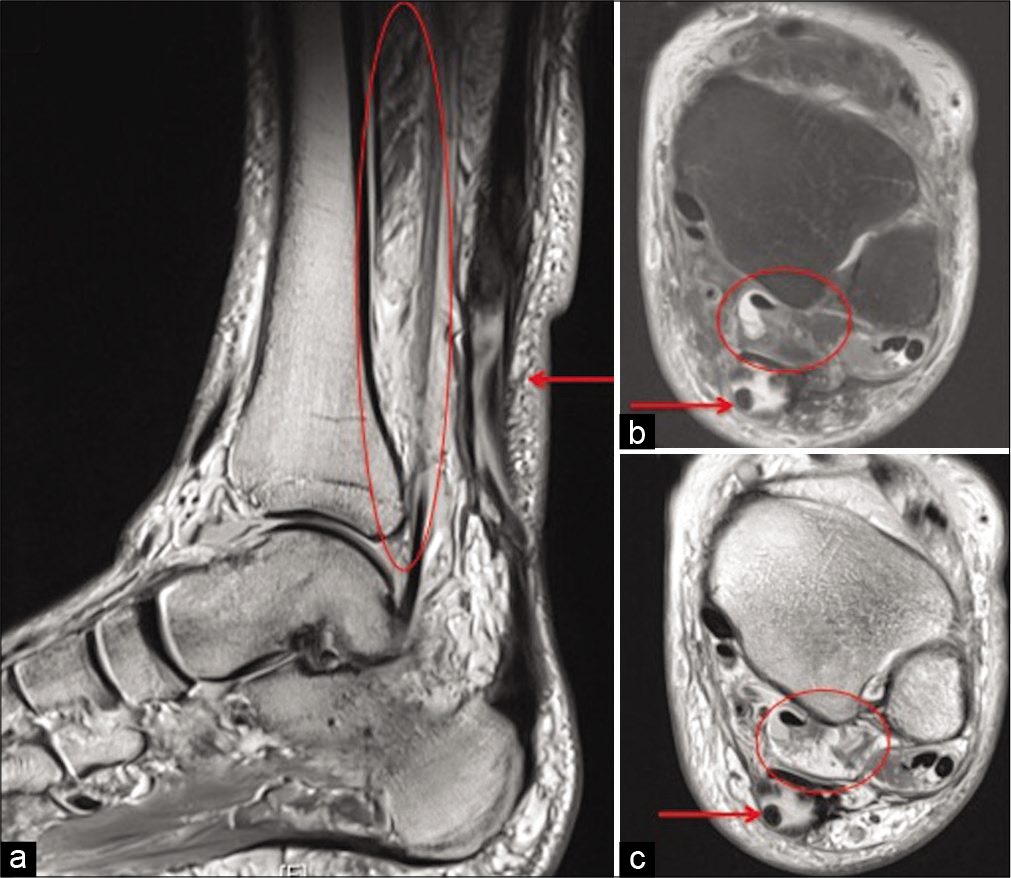
- Sagittal PD (a), axial PDFS (b), and PD (c) show chronic full thickness rupture of Achilles tendon (arrow) and marked fatty atrophy of flexor hallucis longus (circle) (Goutallier Grade 4).
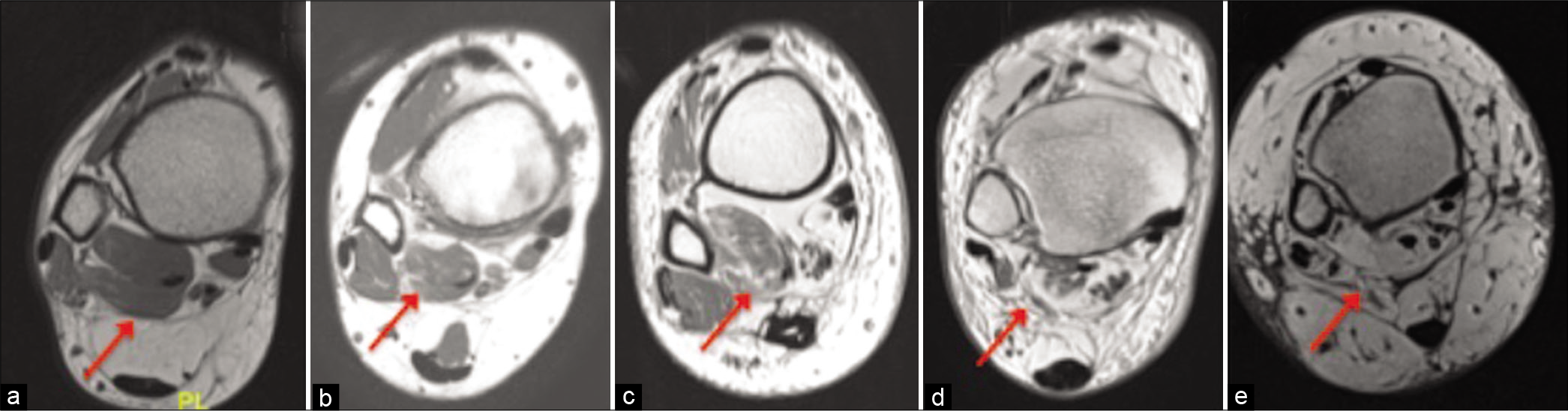
- Axial PD showing Goutallier Grade 0 (a), 1 (b), 2 (c), 3 (d), and 4 (e) of flexor hallucis longus.
Other Pathologies of FHL
Posterior ankle impingement is due to restriction of movement at the posterior aspect of the ankle, which results in pain. Symptoms arise due to the repeated compression of bony or soft-tissue structures between the superior aspect of the calcaneum and the posterior aspect of the tibia. It may arise secondary to an acute episode of traumatic hyper-plantar flexion or in the setting of repetitive trauma associated with hyper plantar flexion such as in ballet dancers, footballers and gymnasts.[19]
There are multiple causes of posterior impingement, which include osseous abnormalities such as os trigonum, osteophytes or a steida process, or soft-tissue abnormalities such as synovitis or scar tissue arising from the posteromedial ankle. Other causes for posterior impingement include accessory muscles such as peroneus quartus or flexor accessories digitorum longus. A low-lying FHL muscle belly may also result in posterior impingement.[20]
Posterior impingement may result in irritation of the FHL tendon leading to stenosing tenosynovitis.[21] This can progress to partial or full thickness tear of FHL [Figure 5]. MRI is the mainstay of diagnosis and can delineate the cause of posterior impingement and allow for assessment of associated structures such as the FHL [Figure 6].
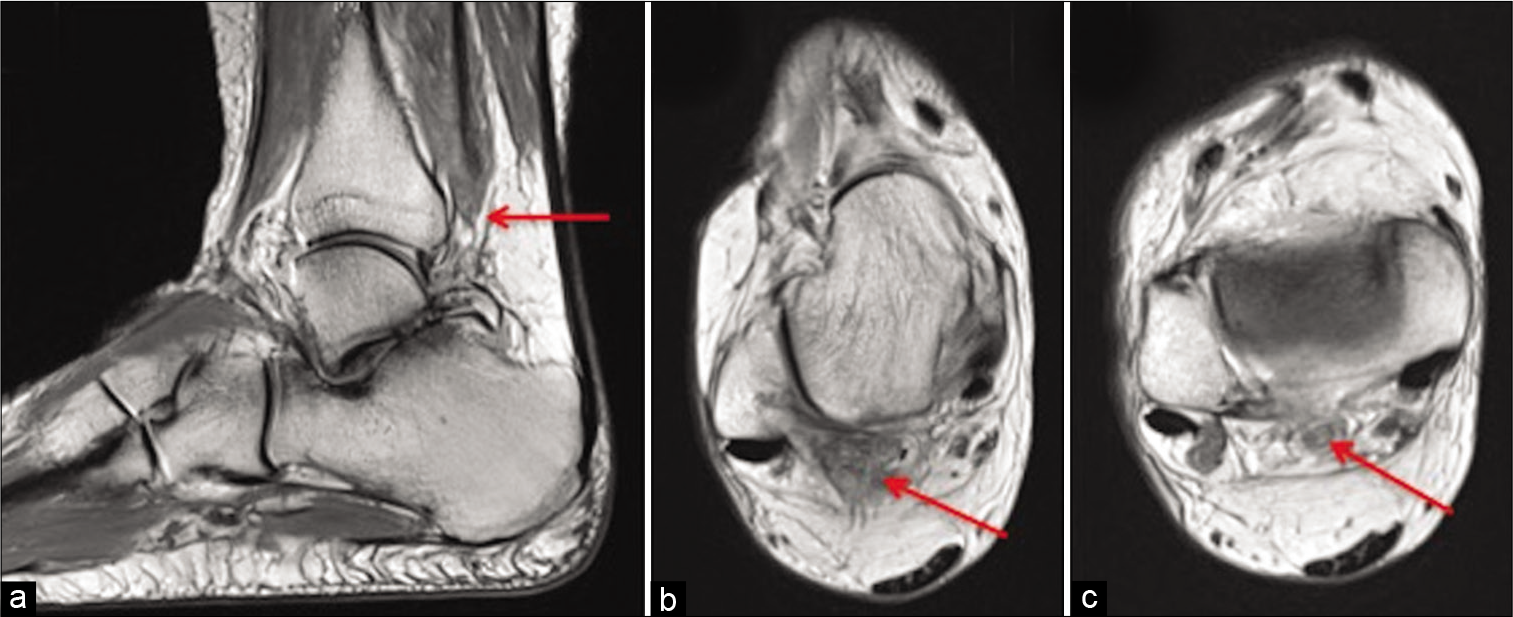
- Sagittal PD (a) and axial PD (b and c) show attenuated tendon of flexor hallucis longus in keeping with high grade near full thickness tear (red arrow).

- Sagittal PD (a) and axial PD (b) show low lying flexor hallucis longus with moderate edema (arrow).
Tenosynovitis is the inflammation of the synovium that surrounds a tendon. It can affect the FHL and is associated with chronic overuse.[22] Stenosing tenosynovitis of the FHL tendon may result as a complication of trauma such as medial malleolar or calcaneal factures, or more commonly due to repetitive micro trauma. This is typically seen in ballet dancers and athletes. Clinical symptoms of tenosynovitis of the FHL classically include posteromedial ankle pain that is triggered by plantar flexion activities.[23]
MR imaging is the main diagnostic imaging modality that is used when tenosynovitis is suspected and plays a key role in defining the abnormal fluid within the FHL tendon sheath. Tenosynovitis is best diagnosed on T2-weighted imaging.[24] The ankle communicates with the FHL tendon sheath in 25% of the normal population and abnormal amounts of fluid in the ankle joint may involve the FHL tendon sheath.[25] Characteristically, tenosynovitis of the FHL is recognized when fluid in the FHL tendon sheath is disproportionate, that is, more fluid in the FHL than in the ankle joint [Figure 7]. Furthermore, the presence of synovitis or prominent stranding aids in the diagnosis.

- Sagittal PDFS (a), coronal PDFS (b), and axial PD (c) show large amount of fluid (circle) in the tendon sheath of flexor hallucis longus which is disproportionate to ankle joint effusion.
Tenosynovitis is usually managed nonoperatively with anti-inflammatory medications along with rest and immobilization in severe cases. If conservative management fails, ultrasound guided steroid injections may aid in symptomatic treatment.[24]
Loose bodies are characterized by benign osseocartilaginous bodies which may be found affecting any part of the body, typically lined by synovial tissue.[26] There is a female to male predominance and the area’s most commonly affected include the knee, followed by the hip joint; however, involvement of the foot and ankle joints has been reported, with several cases involving the FHL tendon sheath specifically.[27-29] Loose bodies found in the FHL tendon sheath are a result of synovial chondromatosis or OA affecting the ankle or the posterior subtalar joint, as the ankle joint and the FHL tendon sheath communicate.[23] Loose bodies track down the tendon sheath to lie behind the ankle or between the talar tubercles (under the sustentaculum tali) up to the master knot of Henry [Figure 8]. They very rarely extend past the master knot of Henry.[30]
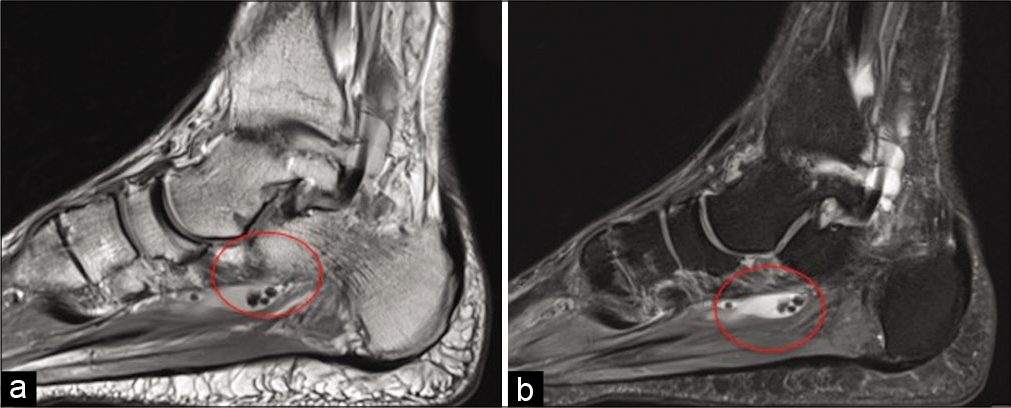
- Sagittal PD (a) and PDFS (b) demonstrate loose bodies (circle) in the tendon sheath of flexor hallucis longus.
Patients usually present with pain, swelling, and restriction of movements of the affected tendon.[31] It is considered a self-limiting condition that eventually leads to an inactive synovium.[32] Whether or not this has any clinical significance is questionable; however, studies have shown that if left untreated, loose bodies have the potential to cause damage and inflammation around the tendon, muscular atrophy, and joint degeneration.[33]
Giant cell tumors of the tendon sheath (GCT-TS) are a benign group of slow-growing tumors that originate from the tendon sheath involved or the surrounding soft-tissue.[34] They commonly occur between 30 and 50 years of age and have a slight female predominance.[35,36] In most cases, GCT-TS present as a slow growing, painless mass. They commonly affect the tendons of the hands and are less frequently encountered in the foot and ankle region.[37,38] The FHL tendon is very rarely involved with only a single case reported in the literature [Figure 9].[39] The treatment of GCTTS depends mainly on pain severity, the size of the tumor and adjacent bone invasion. This is due to the difficulty in surgical excision, which is often incomplete and is associated with high recurrence rates.[40,41]
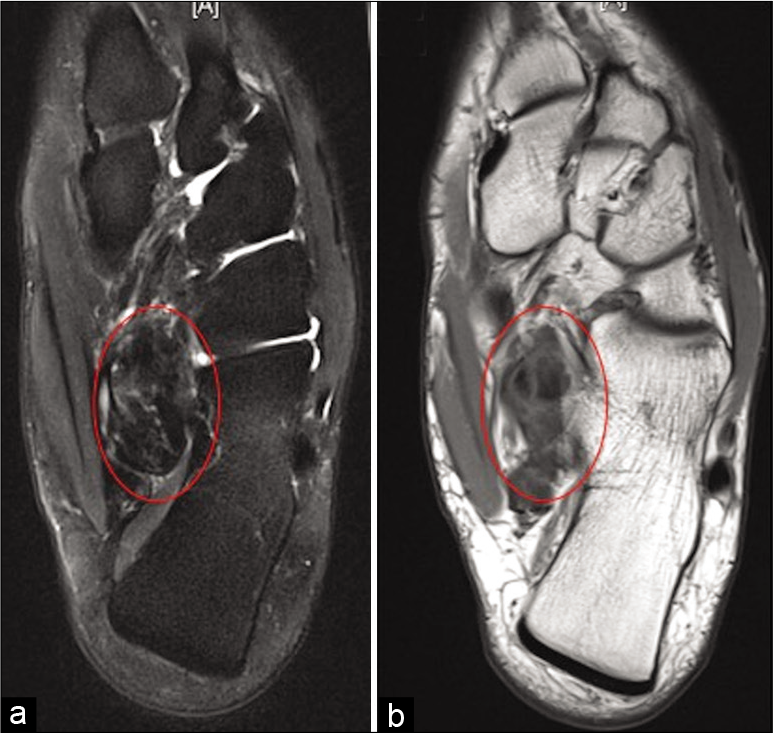
- Axial PDFS (a), PD (b), and coronal PDFS (c) show low signal lesion in relation to flexor hallucis longus at the level of master knot of Henry in keeping with giant cell tumor of tendon sheath (red circle).
If surgery does become a necessity, early planning is important and imaging diagnosis is essential.[39] Many different imaging modalities have been used to aid in the diagnoses GCT-TS.[42] Ultrasonography, which is usually the first-line method of diagnosis, would demonstrate a solid homogenous hypoechoic mass and can also provide information regarding the tumor size and vascularity.[43] The gold standard imaging modality used for diagnosis, however, is MR imaging.
From our data base, we found one GCT-TS affecting the FHL tendon adjacent to the level of the master knot of Henry.
CONCLUSION
We describe a spectrum of pathologies that may involve the FHL. We recommend a preoperative radiological (MR) assessment of the FHL tendon/muscle to establish that the FHL tendon/muscle is suitable for transfer surgery.
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Toe flexor strength, flexibility and function and flexor hallucis longus tendon morphology in dancers and non-dancers. Med Probl Perform Art. 2015;30:152-6.
- [CrossRef] [PubMed] [Google Scholar]
- Trauma to the heel cord In: Jahss MH, ed. Disorders of the Foot and Ankle. Philadelphia, PA: WB Saunders; 1991. p. :2357.
- [Google Scholar]
- Chronic rupture of tendo Achillis. Foot Ankle Clin. 2007;12:583-96.
- [CrossRef] [PubMed] [Google Scholar]
- Attenuation of skeletal muscle and strength in the elderly: The health ABC study. J Appl Physiol 1985. 2001;90:2157-65.
- [CrossRef] [PubMed] [Google Scholar]
- Synovial fluid in the hind-foot and ankle: Detection of amount and distribution with US. Radiology. 1995;197:275-8.
- [CrossRef] [PubMed] [Google Scholar]
- Fluid in normal and abnormal ankle joints: Amount and distribution as seen on MR images. AJR Am J Roentgenol. 1994;162:111-4.
- [CrossRef] [PubMed] [Google Scholar]
- Aging-associated exacerbation in fatty degeneration and infiltration after rotator cuff tear. J Shoulder Elbow Surg. 2014;23:99-108.
- [CrossRef] [PubMed] [Google Scholar]
- Skeletal muscle disease: Patterns of MRI appearances. Br J Radiol. 2012;85:e1298-308.
- [CrossRef] [PubMed] [Google Scholar]
- Factors associated with clinical and structural outcomes after arthroscopic rotator cuff repair with a suture bridge technique in medium, large, and massive tears. J Shoulder Elbow Surg. 2014;23:1675-81.
- [CrossRef] [PubMed] [Google Scholar]
- Fatty degeneration and atrophy of the rotator cuff muscles after arthroscopic repair: Does it improve, halt or deteriorate? Arch Orthop Trauma Surg. 2014;134:985-90.
- [CrossRef] [PubMed] [Google Scholar]
- Rupture of the sub-scapularis tendon (isolated or in combination with supraspinatus tear): When is a repair indicated? J Shoulder Elbow Surg. 2006;15:659-64.
- [CrossRef] [PubMed] [Google Scholar]
- Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr. 2005;82:1210-7.
- [CrossRef] [PubMed] [Google Scholar]
- Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372-9.
- [CrossRef] [PubMed] [Google Scholar]
- Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885-92.
- [CrossRef] [PubMed] [Google Scholar]
- MRI for the demonstration of subclinical muscle involvement in muscular dystrophy. Clin Radiol. 2007;62:160-5.
- [CrossRef] [PubMed] [Google Scholar]
- Fatty degeneration of the muscles of the rotator cuff: Assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8:599-605.
- [CrossRef] [Google Scholar]
- Fatty muscle degeneration in cuff ruptures. Pre-and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78-83.
- [CrossRef] [Google Scholar]
- Posterior ankle impingement in athletes: Pathogenesis, imaging features and differential diagnoses. Eur J Radiol. 2015;84:2231-41.
- [CrossRef] [PubMed] [Google Scholar]
- MRI features of posterior ankle impingement syndrome in ballet dancers: A review of 25 cases. Clin Radiol. 2004;59:1025-33.
- [CrossRef] [PubMed] [Google Scholar]
- Stenosing tenosynovitis of the flexor hallucis longus tendon and posterior impingement upon the os trigonum in ballet dancers. Foot Ankle. 1982;3:74-80.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented villonodular synovitis, bursitis and tenosynovitis. Arch Pathol. 1941;131:731-65.
- [Google Scholar]
- Tenosynovitis of the flexor hallucis longus: A clinical study of the spectrum of presentation and treatment. Foot Ankle Int. 2005;26:291-303.
- [CrossRef] [PubMed] [Google Scholar]
- Tenosynovitis of the flexor hallucis longus in a long-distance runner. Med Sci Sports Exerc. 1996;28:277-9.
- [CrossRef] [PubMed] [Google Scholar]
- Stenosing tenosynovitis of the flexor hallucis longus tendon at the great toe. Foot Ankle. 1981;2:46-8.
- [CrossRef] [PubMed] [Google Scholar]
- Tenosynovial osteochondromatosis of the flexor hallucis longus tendon treated by tendoscopy. J Foot Ankle Surg. 2015;54:758-64.
- [CrossRef] [PubMed] [Google Scholar]
- Tenosynovial chondromatosis of the shoulder. Bull Hosp Jt Dis Orthop Inst. 1981;41:37-47.
- [Google Scholar]
- Tenosynovial osteochondromatosis of the tarsal tunnel. Skeletal Radiol. 2003;32:99-102.
- [CrossRef] [PubMed] [Google Scholar]
- Synovial chondromatosis of the subtalar joint and tenosynovial chondromatosis of the posterior ankle. J Am Podiatr Med Assoc. 2006;96:59-62.
- [CrossRef] [PubMed] [Google Scholar]
- Flexor hallucis longus loose bodies-an unusual cause of plantar midfoot pain. OMICS J Radiol. 2013;2:152.
- [CrossRef] [Google Scholar]
- Endoscopic loose body removal from zone 2 flexor hallucis longus tendon sheath. Arthrosc Tech. 2016;5:e465-9.
- [CrossRef] [PubMed] [Google Scholar]
- Extra-articular synovial chondromatosis. J Bone Joint Surg Am. 1962;44:77-86.
- [CrossRef] [Google Scholar]
- Arthroscopic synovectomy, removal of loose bodies and selective biceps tenodesis for synovial chondromatosis of the shoulder. J Bone Joint Surg Br. 2007;89:1329-35.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of tendon sheath: Case series and review of literature. J Hand Microsurg. 2010;2:67-71.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumour of the tendon sheath of the hand. J Orthop Surg (Hong Kong). 2011;19:218-20.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumour of tendon sheath (localised nodular tenosynovitis): Clinicopathological features of 71 cases. J Clin Pathol. 2001;54:404-7.
- [CrossRef] [PubMed] [Google Scholar]
- Fibrous xanthoma of synovium (giant-cell tumor of tendon sheath, pigmented nodular synovitis). A study of one hundred and eighteen cases. J Bone Joint Surg Am. 1969;51:76-86.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of the tendon sheath (nodular tenosynovitis). A study of 207 cases to compare the large joint group with the common digit group. Cancer. 1986;57:875-84.
- [CrossRef] [Google Scholar]
- Giant cell tumor of the flexor hallucis longus tendon sheath: A case study. J Am Podiatr Med Assoc. 2011;101:187-9.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of the tendon sheath in the foot and ankle: Case series and review of the literature. J Foot Ankle Surg. 2013;52:24-7.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of tendon sheath: Study of 64 cases and review of literature. G Chir. 2013;34:149-52.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumors of the tendon sheath may present radiologically as intrinsic osseous lesions. Eur Radiol. 2007;17:499-502.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumors of the tendon sheath: Analysis of sonographic findings. AJR Am J Roentgenol. 2004;183:337-9.
- [CrossRef] [PubMed] [Google Scholar]






