Translate this page into:
Glycemic control and adverse effects in patients with type 2 diabetes receiving basal-bolus insulin regimen versus premixed insulin regimen: An observational study
*Corresponding author: Prithwis Mitra, Department of Pharmacology, Institute of Post-Graduate Medical Education and Research and Seth Sukhlal Karnani Memorial Hospital, Kolkata, West Bengal, India. prithwis923@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mitra P, Siddhanta S, Yasmin N, Sengupta G. Glycemic control and adverse effects in patients with type 2 diabetes receiving basal-bolus insulin regimen versus premixed insulin regimen: An observational study. Indian J Med Sci. 2024;76:56-60. doi: 10.25259/IJMS_204_2023
Abstract
Objectives:
Several studies have compared the basal-bolus (BB) and premixed (PM) insulin regimens with varying results. This study aimed to evaluate the glycemic control and occurrence of hypoglycemia with these regimens in insulin-naïve patients with type 2 diabetes mellitus (T2D) in the Indian subpopulation.
Materials and Methods:
This was a 12-week (wk) prospective, observational study in 60 adult patients (distributed 1:1) with >7 years of T2D and uncontrolled with three oral drugs. Changes in glycemic parameters at wk4 and wk12 were assessed, and hypoglycemia events were also recorded.
Results:
The PM insulin showed a significant decrease in fasting plasma glucose (FPG) at wk4 from baseline (P = 0.02) and at wk12 (P < 0.001), while in the BB insulin group, the change was significant only at wk12 (P < 0.0001). There were greater reductions in the PM group in FPG at wk4 (PM vs. BB: P = 0.04) and wk12 (P = 0.03) compared to the BB group. The post-prandial plasma glucose in both groups significantly reduced from baseline at wk4 (PM group P = 0.034; BB group; P = 0.034) and wk12 (PM group P < 0.0001 and BB group: P < 0.0001). However, there were no between-group differences at wk4 (P = 0.12) but only at wk12 (P = 0.009) with greater reductions in the PM group. The PM group showed a slightly greater reduction in glycated hemoglobin versus the BB group (9.18% vs. 7.08%; P = 0.39). There was no significant difference (P = 0.49) in the incidence of hypoglycemia.
Conclusion:
Both treatments significantly improved glycemic control and were not associated with any severe episodes of hypoglycemia. Therefore, the choice should instead be guided by the insulin-related (posology, complexity) and patient-related (dietary habits, adherence levels) factors.
Keywords
Premixed insulin
Basal-bolus insulin
Insulin therapy initiation
Insulin naïve
Glycated hemoglobin
Hypoglycemia
INTRODUCTION
Insulin therapy forms the cornerstone of diabetes management in the majority of the population with type 2 diabetes mellitus (T2D) due to its chronic and progressive nature.[1-4] The choice of insulin is dictated by the meal content and pattern of the patient and anticipated adherence and compliance to insulin therapy, along with drug interactions with various pre-existing oral hypoglycemic agents (OHAs) used. The choice is also guided by the complexity of the regimen, which affects the adherence of the patient.[4,5]
The American Association of Clinical Endocrinologists guideline 2022, International Diabetes Federation guidelines 2017, and The Research Society for the Study of Diabetes in India 2019 recommend initiation of insulin therapy with either basal or premix insulin, while the Indian National Consensus Group 2013 recommends initiation with only premix insulin.[6-9] Clinical evidence in Asian patients suggests that there is a subgroup of the Asian patient population with T2D with high glycated hemoglobin (HbA1c) (>8.5%) levels in whom basal insulin may not be sufficient to achieve glycemic goals. In this subgroup of insulin-naïve patients, an aggressive dual pharmacotherapy using basal and prandial insulin that aims at both fasting and post-prandial glucose levels should be preferred. Premixed (PM) insulin therapy (70/30 insulin [a mixture consisting of 70% intermediate-acting and 30% regular insulin]) is appropriate for patients who cannot count carbohydrates or those who have constant eating patterns and a uniform lifestyle. The basal-bolus (BB) regimen mimics the physiological insulin secretion from the pancreas; however, this insulin type needs frequent and active self-monitoring. Compared to PM, BB requires frequent dosing, which may negatively affect patient adherence.[5,10]
Several studies have compared the efficacy of these two insulin regimens.[5] Few are in favor of either, while some demonstrated similar glycemic control and HbA1c lowering.[11-13] Studies have also found differences in the incidence of hypoglycemia between BB and PM.[14] Interestingly, none of the studies have compared the efficacy and tolerability of these two insulin regimens in Indian insulin-naïve patients with diabetes. Therefore, we undertook this study to evaluate glycemic control associated with the use of either a BB insulin regimen or PM insulin regimen in the insulin-naïve Indian subpopulation.
MATERIALS AND METHODS
Study design
This 12-week (wk), prospective, observational study was conducted in patients with type 2 diabetes with longitudinal follow-up at wk 4 and wk 12. The patients included were aged between 18 and 65 years, uncontrolled on lifestyle modification and three oral hypoglycemic medications (as per the treating clinician’s opinion) with HbA1c > 8. The patients who agreed to be initiated on either BB or in a PM insulin regimen and provided informed consent were included in the study. Patients receiving medications, that is, steroids, thiazides, and beta blockers, which interfere with blood sugar level, were pregnant and/or lactating were excluded from the study. Furthermore, subjects with comorbid chronic liver or kidney disease, other serious concomitant disease, acute illness, or a history of alcohol abuse were not included in the study.
The age, gender, height, weight, waist circumference, hip circumference, and blood pressure in sitting posture were determined. The body mass indices and waist-to-hip ratio were calculated. Change in fasting plasma glucose (FPG), prandial plasma glucose (PPG), and HbA1c% at wk 4 and wk 12 with either regimen was assessed as an indicator of efficacy. Hypoglycemia was identified and classified using the American Diabetes Association and European Association for the Study of Diabetes classification as shown below: [15,16] (i) Hypoglycemia alert value (level 1) ≤70 mg/dL (3.9 mmol/L), sufficiently low for treatment with fast-acting carbohydrate and dose adjustment of glucose-lowering therapy; (ii) Clinically significant hypoglycemia (level 2) <54 mg/dL (3.0 mmol/L), sufficiently low to indicate serious, clinically important hypoglycemia; (iii) Severe hypoglycemia (level 3), no specific glucose threshold, hypoglycemia associated with severe cognitive impairment requiring external assistance for recovery.
Both groups received standard treatment and care. Most patients were on combinations of metformin, glimepiride, and vildagliptin, which was continued unchanged throughout the study at clinicians’ discretion. Therapy for pre-existent comorbidities such as hypertension, dyslipidemia, ischemic heart disease, hypothyroidism, bronchial asthma, chronic obstructive pulmonary disease, and other disorders was continued as per the direction of the attending clinician.
The PM regimen involves two doses of PM insulin, that is, two injections per day. In the BB regimen, a single dose of long-acting peakless insulin was combined with three doses of regular insulin and involved four injections per day.
Ethical considerations
The study conformed to the Declaration of Helsinki for biomedical research involving human subjects. The study had been registered in the Clinical Trial Registry.
Statistical analysis
After collecting the data, it was transcribed in MS Excel Datasheet and analyzed using GraphPad Prism version 8.4.2 statistical software (GraphPad Software Inc., San Diego, CA, USA). The numeric study variables were summarized and expressed as Mean ± Standard Deviation. Categorical variables were expressed as percentages. The test for normality was done using the Shapiro–Wilk test. Intergroup comparison of numeric parametric data was done by unpaired t-test. The intragroup comparison was made using the Kruskal-Wallis test. A value of P ≤ 0.05 was considered statistically significant, along with a 95% confidence interval.
RESULTS
The study included 60 patients based on the inclusion criteria and allocated in a ratio of 1:1 to BB or PM insulin. The demographic details are depicted in Table 1. None of the baseline data differed significantly between the groups except PPG, which was significantly greater in the PM regimen group.
| Parameter | Premixed regimen (n=30) |
Basal-bolus regimen (n=30) |
P-value |
|---|---|---|---|
| *Age (years) | 52.43±8.60 | 54.43±10.15 | 0.41 |
| Male | 13 (43.33%) | 18 (60%) | 0.30 |
| Female | 17 (56.66%) | 12 (40%) | |
| *Duration of T2DM (years) | 7.90±3.06 | 7.46±4.15 | 0.33 |
| #Family history | 22 (73%) | 24 (80%) | 0.99 |
| #Hypertension | 13 (43.33%) | 15 (50%) | 0.79 |
| #Dyslipidemia | 8 (26.66%) | 10 (33.33%) | 0.77 |
| *Weight (kg) | 64.47±12.98 | 67.67±9.10 | 0.27 |
| *BMI | 25.62±4.44 | 24.63±4.48 | 0.39 |
| *Fasting plasma Glucose (mg/dL) | 182.06±49.30 | 191.40±44.40 | 0.46 |
| *Post-prandial plasma Glucose (mg/dL) | 253.46±54.30 | 234.10±66.20 | 0.03 |
| *HbA1c% | 8.38±1.05 | 7.90±0.78 | 0.11 |
Effect on glycemic parameters
Both insulin regimens reduced FPG and PPG significantly compared to baseline across 12 wks. However, PM insulin showed a significant decrease at wk 4 (from baseline 182.06 ± 49.30 to 146.8 ± 30.77, P = 0.02) up to wk 12 (from baseline 182.06 ± 49.30 mg/dL to 131.6 ± 19.41 mg/dL; P < 0.001), while BB insulin the change in FPG was significant only at wk 12 (from baseline 191.43 ± 44.46 mg/dL to 141.26 ± 15.17 mg/dL; P < 0.0001). There was a significant difference in the FPG between the PM and BB groups at wk 4 (146.80 ± 30.77 mg/dL vs. 167.80 ± 33.14 mg/dL; P = 0.04) and wk 12 (131.60 ± 19.41 mg/dL vs 141.30 ± 15.71 mg/dL; P = 0.03) [Figure 1a].
The PPG in both groups was significantly reduced from baseline at wk 4 ([PM group: from 253.46 ± 54.30 mg/dL to 190.3 ± 32.47 mg/dL; P = 0.034] [BB group: from 234.1 ± 66.20 mg/dL to 190.3 ± 22.47 mg/dL; P = 0.034]) and wk 12 ([PM group: from 253.46 ± 54.30 mg/dL to 171.5 ± 16.62 mg/dL; P < 0.0001] and [BB: 234.1 ± to 158.7 ± 20.21 mg/dL; P < 0.0001]) [Figure 1b]. However, there were no between-group differences at wk 4 (177.70 ± 24.24 mg/dL vs. 190.30 ± 32.47 mg/dL; P = 0.12) but only at wk 12 (158.70 ± 20.21 mg/dL vs. 171.50 ± 16.62 mg/dL; P = 0.009).
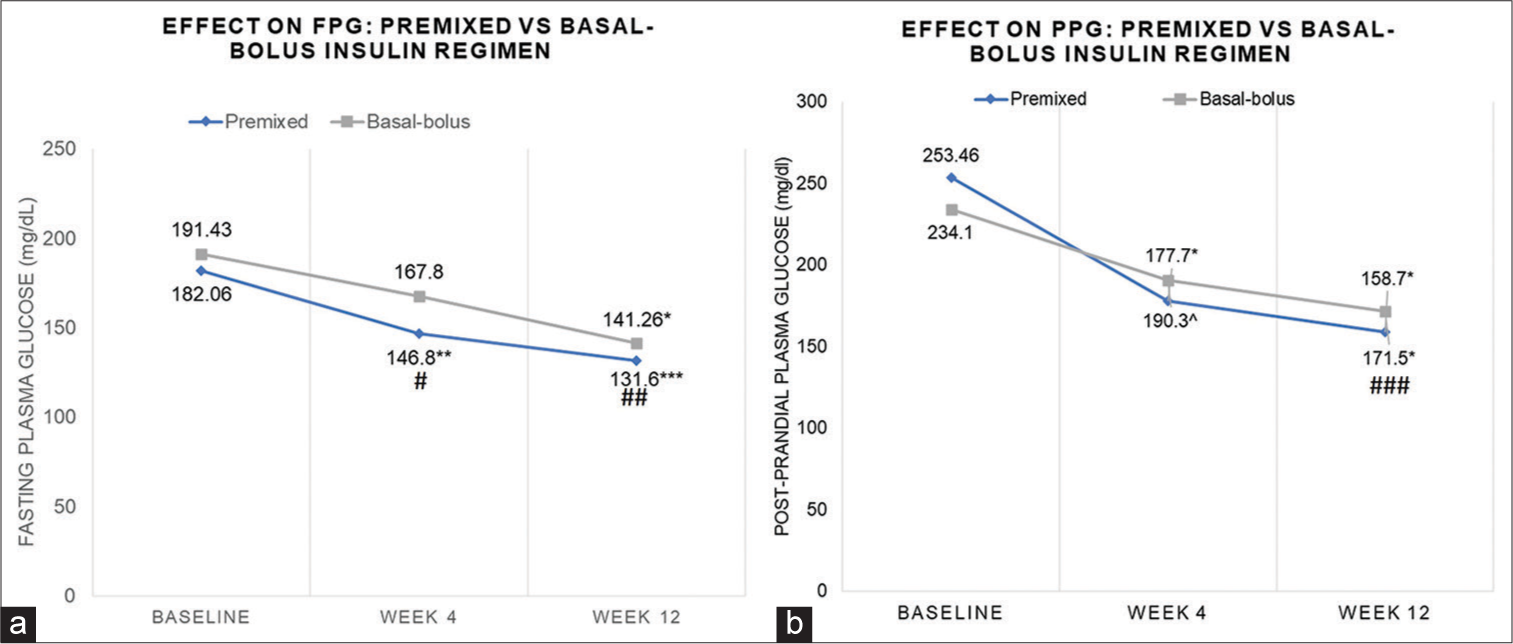
- Effect on (a) Fasting Plasma Glucose (FPG) (b) Post-prandial plasma glucose (PPG): Premixed insulin and Basal-bolus insulin regimen. *P < 0.0001, ^P < 0.034, **P = 0.02. ***P < 0.001 vs baseline, #P = 0.04; ##P = 0.03; ###P = 009 vs Basal-bolus insulin regimen.
In the PM regimen, the mean HbA1c value decreased from 8.38% ± 1.08% at baseline to 7.61% ± 0.61%; P = 0.0002 over 12 wk s with a percentage change of 9.18%. For the patients on the BB regimen, the mean HbA1c changed from 7.90% ± 0.78 to 7.34% ± 0.63%; P = 0.0008 over 12 wk s with a percentage change of 7.08%. Hence, the PM insulin regimen shows a greater percentage reduction in HbA1c compared to the BB regimen; however, the difference between the two HbA1c reductions were not statistically significant (P = 0.39).
Hypoglycemic events
In patients treated with a PM insulin regimen, the incidence of level 1 hypoglycemia was 6.67%, which was self-managed by the patients and did not require medical attention or hospitalization. However, there were no events of hypoglycemia reported by patients managed with BB insulin. There was no significant difference (P = 0.49) in the incidence of hypoglycemia between the two groups.
DISCUSSION
This 12-wk observational study in adult ambulatory subjects with T2D uncontrolled on triple oral hypoglycemic therapy comparing PM and BB insulin regimens demonstrated similar efficacy between the two regimens. PM insulin demonstrated a rapid glycemic control compared to BB, which was more gradual in action. Therefore, glycemic control was achieved earlier (wk 4) with a PM regimen. The drastic glycemic control with PM is also corroborated by the non-serious hypoglycemic events in this group, though only restricted to a few. Overall, at 12 wks, HbA1C change was significant from baseline but not significantly different between groups. The differences noted in the FPG and PPBG could be due to some differences in the baseline values.
The results of our study are similar to those reported by Bellido et al. in hospitalized patients who, too, demonstrated similar glycemic control with either regimen but with a significantly higher incidence of hypoglycemia in the PM arm.[17] A real-world study in Asian patients showed that BB insulin was associated with a greater incidence of hypoglycemia compared to PM regimens, while both cohorts demonstrated clinically meaningful reductions in HbA1c during follow-up. Moreover, after initiation, most patients on PM regimens continued, while several patients switched from BB to less intensive regimens.[18] This could be attributed to PM insulin analogs being a simplified and convenient alternative with a lower frequency of daily injections for patients with T2D who are either unwilling or unable to use BB insulin.[10] The 6-month DURABLE study reported comparable reductions in A1C and similar incidence of hypoglycemia and proportion of weight gain.[19] A recent 1-year study in veterans with T2D found no significant difference between glycemic control and hypoglycemia.[20] A crossover study found higher weight gain with BB insulin regimen than PM, while the incidence of hypoglycemia was comparable.[21] A systematic review of eight studies comparing randomized controlled trial and real-world data from primary care also reported that both insulin regimens were associated with HbA1c reduction. Yet, there were no significant differences between BB and premix in either type of study after adjustment for age and baseline weight.[22] These data, thus, underscore the importance of patient factors that would influence the probable efficacy of PM over BB rather than slightly varying results for endpoints such as HbA1C, hypoglycemia, or body weight.[22] Thus, the choice of insulin should be guided by patient factors since the efficacy and safety of these two insulin regimens were almost comparable.
Insulin premixes may be preferred for patients in need of both components of treatment (basal and bolus) but perceive the use of the BB regimen as complex.[10] A questionnaire survey reported that adherence in terms of skipping insulin doses was better with PM than BB insulin.[23] More than twice as many of those on BB skipped insulin than those on PM. Furthermore, nearly thrice as many patients who skipped more than one dose were on BB compared to a PM insulin regimen. Thus, a BB regimen was associated with inferior constancy and adherence to daily insulin injections. The primary reasons cited were an obstruction to their daily routine, distraction by social commitments, and a busy schedule.[23] Moreover, it has also been reported that nearly two-thirds of the patients were initiated on BB regimens as inpatients, while approximately one-third of patients initiating PM regimens were inpatients. Thus, the clinical evidence suggests that these findings are in tandem with the belief that PM therapy is a simpler and more manageable alternative to BB therapy.[23] Probably due to the ease of use and optimal glycemic control, in many countries globally, PM insulin is among the most commonly prescribed formulations in patients with T2D.[17]
Limitations
The present study was an observational one with its peculiar limitations of lack of absolute control over the medication administration and other confounding factors. Besides the small sample size, the study was limited by the lack of data recorded for meal timing and content, adherence, and patient skills in terms of self-management of diabetes (self-monitoring, dose titration) and estimate of adherence to the prescribed insulin regimen. A comparison of these would have provided additional supportive evidence for factors to be considered when initiating insulin based on patient factors.
However, the population was consistent in terms of prior oral antidiabetic regimen and duration of diabetes, which improved the reliability of the data obtained.
CONCLUSION
Both treatments significantly improved glycemic control and were not associated with any severe episodes of hypoglycemia. Between the PM and BB insulin regimens, PM should be preferred for patients with consistent dietary habits and a high risk of non-adherence due to difficulty in understanding complex regimens. In comparison, the BB may be preferred in patients who are skilled at self-management of diabetes since it requires active and frequent self-monitoring and dose titration to meet glycemic targets.
Acknowledgment
We want to acknowledge Seema Kalel, Intersect Communications, for drafting the manuscript.
Ethical approval
The research/study approved by the Institutional Ethics Committee vide number IPGME&R/IEC/2021/076, dated 6th February 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- International Diabetes Federation. 2021. Available from: https://diabetesatlas.org/idfawp/resource-files/2021/11/IDFDA10-global-fact-sheet.pdf [Last accessed on 2023 Feb 24]
- [Google Scholar]
- Epidemiology of Type 2 diabetes in India. Indian J Ophthalmol. 2021;69:2932-8.
- [CrossRef] [PubMed] [Google Scholar]
- World Health Organization fact sheet diabetes. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes [Last accessed on 2023 Feb 24]
- [Google Scholar]
- Association of glycaemia with macrovascular and microvascular complications of Type 2 diabetes (UKPDS 35): Prospective observational study. BMJ. 2000;321:405-12.
- [CrossRef] [PubMed] [Google Scholar]
- Different insulin initiation regimens in patients with Type 2 diabetes-a review article. Int J Diabetes Clin Res. 2018;5:83.
- [CrossRef] [Google Scholar]
- American association of clinical endocrinology clinical practice guideline: Developing a diabetes mellitus comprehensive care plan-2022 update. Endocr Pract. 2022;28:923-1049.
- [CrossRef] [PubMed] [Google Scholar]
- RSSDI consensus recommendations on insulin therapy in the management of diabetes. Int J Diabetes Dev Ctries. 2019;39:43-92.
- [CrossRef] [Google Scholar]
- Consensus on initiation and intensification of premix insulin in Type 2 diabetes management. J Assoc Physicians India. 2017;65:59-73.
- [Google Scholar]
- 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2022. Diabetes Care. 2022;45:S125-43.
- [CrossRef] [PubMed] [Google Scholar]
- Role of premixed insulin analogues in the treatment of patients with Type 2 diabetes mellitus: A narrative review. J Diabetes. 2014;6:100-10.
- [CrossRef] [PubMed] [Google Scholar]
- Prandial premixed insulin analogue regimens versus basal insulin analogue regimens in the management of Type 2 diabetes: An evidence-based comparison. Clin Ther. 2007;29:1254-70.
- [CrossRef] [Google Scholar]
- Glycemic control with insulin glargine plus insulin glulisine versus premixed insulin analogues in real-world practices: A cost-effectiveness study with a randomized pragmatic trial design. Clin Ther. 2011;33:841-50.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison between a basal-bolus and a premixed insulin regimen in individuals with type 2 diabetes-results of the GINGER study. Diabetes Obes Metab. 2010;12:115-23.
- [CrossRef] [PubMed] [Google Scholar]
- Pre-mixed insulin has a similar efficacy to basal-bolus insulin in reducing HbA1c levels in Type 2 diabetics. Clin Res Pract. 2021;7:eP2472.
- [CrossRef] [Google Scholar]
- 6. Glycemic targets: Standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S55-64.
- [CrossRef] [PubMed] [Google Scholar]
- Glucose concentrations of less than 3.0 mmol/l (54 mg/dl) should be reported in clinical trials: A joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2017;60:3-6.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of basal-bolus and premixed insulin regimens in hospitalized patients with Type 2 diabetes. Diabetes Care. 2015;38:2211-6.
- [CrossRef] [PubMed] [Google Scholar]
- A real-world, observational study of the initiation, use, and effectiveness of basal-bolus or premixed insulin in Japanese people with Type 2 diabetes. Diabetes Ther. 2021;12:1341-57.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized, open-label, parallel-group evaluations of basal-bolus therapy versus insulin lispro premixed therapy in patients with Type 2 diabetes mellitus failing to achieve control with starter insulin treatment and continuing oral antihyperglycemic drugs: A noninferiority intensification substudy of the DURABLE trial. Clin Ther. 2010;32:896-908.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetes-related outcomes with basal-bolus vs. premixed insulin among veterans with Type 2 diabetes: A single institutional retrospective study. Am J Med Sci. 2023;366:38-43.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative effectiveness of basal-bolus versus premix analog insulin on glycemic variability and patient-centered outcomes during insulin intensification in Type 1 and Type 2 diabetes: A randomized, controlled, crossover trial. J Clin Endocrinol Metab. 2012;97:3504-14.
- [CrossRef] [PubMed] [Google Scholar]
- Premixed vs basal-bolus insulin regimen in Type 2 diabetes: Comparison of clinical outcomes from randomized controlled trials and real-world data. Diabet Med. 2017;34:1728-36.
- [CrossRef] [PubMed] [Google Scholar]
- Adherence to insulin treatment in insulin-naïve Type 2 diabetic patients initiated on different insulin regimens. Patient Prefer Adherence. 2015;9:1225-31.
- [CrossRef] [PubMed] [Google Scholar]







