Translate this page into:
Goodpasture’s syndrome: A case series of unusual presentations including in a pregnancy
*Corresponding author: Rajesh Jhorawat, Department of Nephrology, All India Institute of Medical Sciences, Jodhpur, Rajasthan, India. jhorawat2000@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chandra GB, Bajpai N, Vishwajeet V, Nalwa A, Yadav T, Chaturvedy M, et al. Goodpasture’s syndrome: A case series of unusual presentations including in a pregnancy. Indian J Med Sci. doi: 10.25259/IJMS_146_2024
Abstract
Anti-glomerular basement membrane disease is a rare but potentially life-threatening autoimmune disease. Here, we present three patients with different clinical profiles. The preserved urine output significantly impacts the renal outcome. Renal involvement with oliguria is the most important clinical indicator of poor outcomes in our cases.
Keywords
Anti-glomerular basement membrane disease
Pregnancy
Goodpasture syndrome
Oliguria
Preserve urine output
INTRODUCTION
Anti-glomerular basement membrane (GBM) disease is potentially lethal autoimmune disease with bimodal distribution characterized by rapidly progressive glomerulonephritis (GN) with glomerular cellular crescents and linear deposits of immunoglobulin G (IgG) along the GBM.[1] Goodpasture syndrome (GS) is characterized by three typical clinical or laboratory findings: pulmonary hemorrhage, GN, and the presence of autoantibodies against the GBM.[2] The predominant pathogenic autoantibody is the IgG isotype. It is directed to epitopes on the non-collagenous (NC1) domain of Type IV collagen in a peptide sequence of the alpha-3 chains.[2] Its frequency has been reported as less than one case per million/year, and untreated patients did not recover renal function and had substantial morbidity. Most of the patients become dialysis dependent gradually.[3] The severity of renal failure at the time of presentation is important for both renal and patient survival, so early diagnosis is crucial for improving outcomes. The plasma exchange (PE) associated with corticosteroids and cyclophosphamide has dramatically improved outcomes.[4] We are describing four unusual presentations of anti-GBM disease.
CASE SERIES
Case 1
A 25-year-old female at 34 weeks of gestation presented with c/o hematuria, abdominal pain, mild pedal edema, renal dysfunction (urea/creatinine of 200/12.8 mg/dL), and decreased urine output that progressed anuria. She started on hemodialysis. Her laboratory investigation highlighted in Table 1. She delivered healthy male baby. However, following her delivery, she developed chest symptoms with respiratory distress and hemoptysis. Her renal biopsy showed circumferential cellular crescents and strong (3+) linear positivity along the GBM for antisera specific for IgG, C3, kappa, and lambda in all glomeruli on immunofluorescence (IF) [Figure 1a and b]. High-resolution computed tomography (HRCT) thorax showed evidence of diffuse alveolar hemorrhage. She was started on pulse steroid and cyclophosphamide and received plasmapheresis until her antibody titer became non-detectable. There was no improvement in kidney function after thirteen sessions of PE, and she remained dialysis-dependent.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age/gender | 25/Female | 22/Male | 30/Female |
| Pregnancy | Yes (34 week of POG) | No | No |
| Duration of illness | 7 days | 16 days | 14 days |
| Smoking | No | No | No |
| Clinical presentation | Hematuria, Abdomen pain, Pedal edema | Fever, Cough, Abdomen pain, Uremic symptoms | Hematuria, Abdomen Pain |
| Urine output | 100–200 mL/day | <100 mL/day | 1.2–1.5/day |
| Baseline S.cr (mg/dl) | 12.86 | 14.76 | 6.28 |
| Urine examination | Protein 2+RBC Full field | Protein 3+RBC-15–16/HPF | Protein-2+RBC-10–15/HPF |
| 24 h Urine Protein | - | - | 1266 mg/g |
| Complement C3 | Normal | Normal | Normal |
| Complement C4 | Normal | Normal | Normal |
| ANA | Negative | Negative | Negative |
| ANCA | Negative | Negative | Negative |
| Anti-GBM | Initially negative After delivery of placenta it became+(14 IU/mL) |
Negative | + |
| Kidney size | Normal | Normal | Normal |
| HRCT chest | DAH+(After delivery of placenta) | DAH+ | Normal |
| Kidney biopsy | Yes (Crescentic GN) IF- Linear IgG 3+, C3 with kappa and lambda |
Yes (Crescentic GN) IF- Linear IgG 3+, C3 with kappa and lambda |
Yes (Crescentic GN) IF- Linear IgG 3+ |
| Total plasma exchange given | Thirteen | Seven | Six |
| Treatment given | Cyclophosphamide+MPS (with oral Pred.) | Cyclophosphamide+MPS (with oral Pred.) | Cyclophosphamide+MPS (with oral Pred.) |
| Follow-Up | |||
| At 1 month | Dialysis dependency | Dialysis dependency | Independent |
| At 6 month | Expired | On MHD | S.Cr- 1.86 mg/dL |
ANA: Anti-nuclear antibody, ANCA: Anti-neutrophilic cytoplasmic antibody, Anti-GBM: Anti-glomerular basement membrane antibody, HRCT: High-resolution computed tomography, DAH: Diffuse alveolar hemorrhage, GN: Glomerulonephritis, IF: Immunofluorescence, MPS: Methylprednisolone, MHD: Maintenance hemodialysis, RBC: Red blood cell, HPF: High-power field, IgG: Immunoglobulin G, POG: Period of gestation, S.Cr: Serum Creatinine
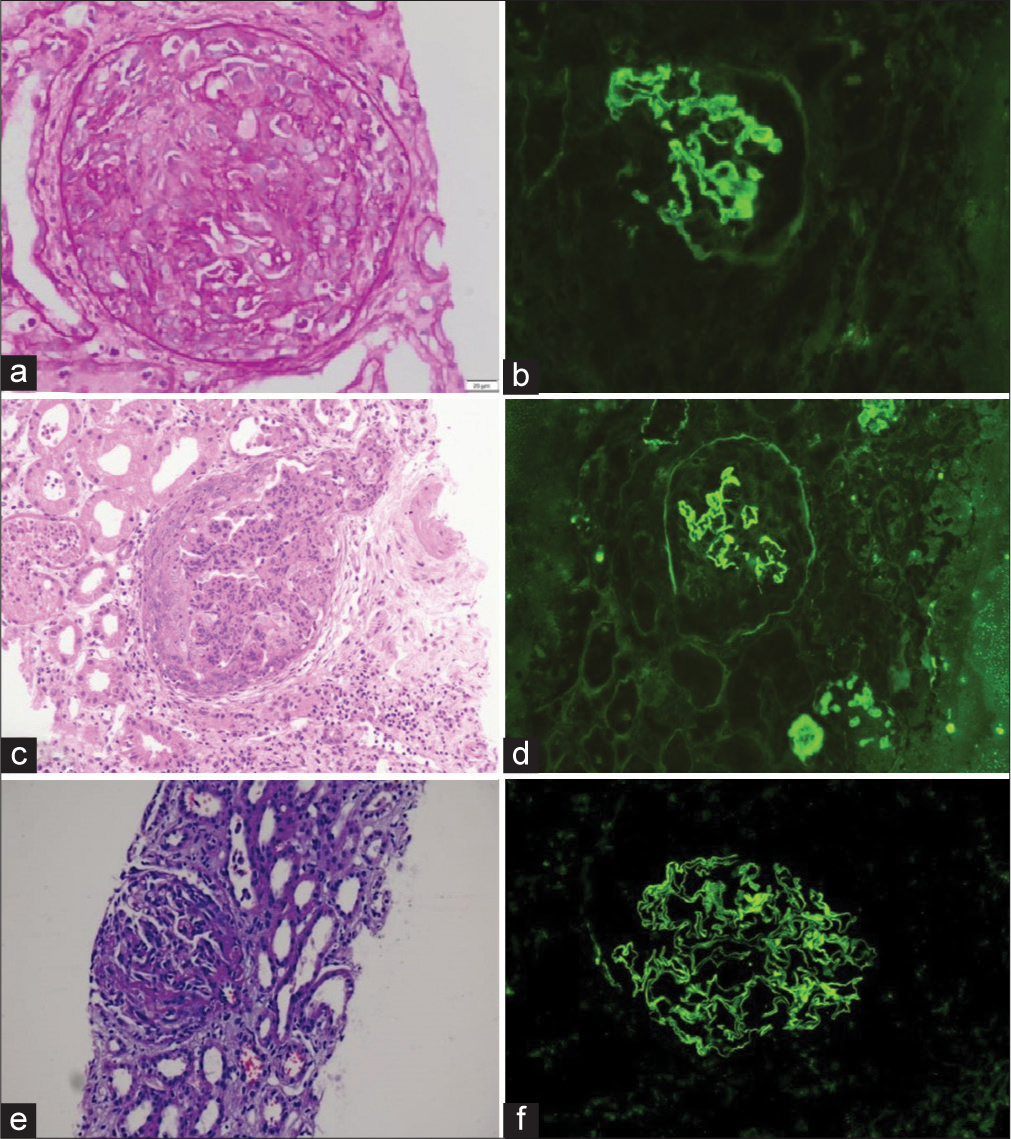
- Case 1: (a) Cellular crescent (PAS ×40) with (b) linear immunoglobulin G (IgG) on immunofluorescence. Case 2: (c) Low-power view shows a cellular crescent with marked neutrophilic exudation in the underlying tuft along with red blood cell casts (Hematoxylin and Eosin ×10). (d) Immunofluorescence shows linear staining of capillary walls and glomerular basement membrane. Case 3: (e) Cellular crescent with fibrinoid necrosis and occasional red blood cell casts (MT ×10) (f) Linear IgG staining on glomerular capillary walls. MT: Masson trichrome stain, PAS: Periodic acid-schiff.
Case 2
A 22-year-old male came with a history of recurrent vomiting, diffuse abdominal pain, bilateral pedal edema, and reduced urine output for 5 days with renal dysfunction of urea of 125 mg/dL and creatinine of 14.87 mg/dL. Her laboratory investigation highlighted in Table 1. Her anti-GBM antibody was negative. Later, he developed a cough and blood-tinged expectoration. Chest X-ray showed infiltration in the left lower zone. His HRCT thorax showed evidence of diffuse alveolar hemorrhage, and bronchial-alveolar lavage showed hemosiderin-laden macrophages. Cyclophosphamide was started with steroids and given seven sessions of PE. Renal biopsy was done, which showed all glomeruli with circumferential fibro-cellular crescents with 30–35% interstitial fibrosis and tubular atrophy and strong 3+ linear positivity along glomerular capillary loops for antisera-specific for IgG, C3, kappa, and lambda on IF [Figure 1c and d]. Repeat anti-GBM titer was repeated; however, it was negative again. He remained dialysis dependent.
Case 3
A 30-year-old female with no known comorbidities presented with abdominal pain and hematuria for 2 weeks . She was not complaining of fever, joint pain, burning micturition, pedal edema, shortness of breath or hemoptysis. She had preserved urine output but deranged renal function (urea of 289 mg/dL, creatinine 6.28 mg/dL) and urine albumin-tocreatinine ratio 1266 mg/g. Her complements (C3 and C4) were normal, anti-GBM was qualitatively positive, and the anti-neutrophilic cytoplasmic antibody panel was negative [Table 1]. Renal biopsy showed crescentic GN featuring cellular crescent 12/15 glomeruli (80%), and IF showed a strong linear positive for IgG [Figure 1e and f]. She received six sessions of PE and steroid pulse for 3 days, followed by oral prednisolone and oral cyclophosphamide 2 mg/kg body weight. Her repeat anti-GBM titers were negative. She responded to the treatment gradually, improved her urine output, and became hemodialysis-free. At present, her creatinine is 1.86 mg/dL.
DISCUSSION
Anti-GBM disease is sporadic in pregnancy, only a few cases have been reported so far, and those reported were either present before pregnancy or during the early trimester. Maternal and fetal outcomes in de novo anti-GBM disease in pregnancy are poor, comparable to systemic lupus erythematosus.[5] Earlier, few GS cases were reported in pregnancy.[2,6-8] Most are nulliparous or primiparous and were in the first or second trimester of gestation at the time of presentation. Three had renal insufficiency and one had an abortion with preterm birth in the remaining. Friend et al. reported recurrent GS in the second pregnancy, leading to permanent renal failure.[9] Riahi et al. reported GS without renal involvement in the third trimester. She was maintained on glucocorticoids without plasmapheresis for 5 weeks before the successful delivery of a live healthy fetus at 39 weeks.[8] In our case, she presented in the third trimester at 34 weeks. Anti-GBM was negative initially, which became positive after delivery of the placenta. It appears that in addition to the kidney, the placenta might act as a sink for anti-GBM antibodies as its level increases after the placental delivery in our case. Fetal complications in de novo anti-GBM disease in pregnancy are high, with prematurity (nearly 100% live births), intrauterine growth restriction (75%), and small for gestational age (75%) and congenital abnormalities (33.3%) live births).[5] In our case, a male baby was delivered prematurely. Anti-GBM levels were not measured in the new-born in our case.
In our second case, the clinical picture is suggestive of GS; however, anti-GBM titers were negative. There was crescentic GN on histopathology with linear staining of IgG with C3 on IF. False-negative tests can be due to (i) low-affinity antibodies, (ii) anti-GBM antibody not easily detected in enzyme-linked immunosorbent assay and radioimmunoassay or radioimmunoassay (esp. IgA or IgG4), (iii) antibody disappearance before the resolution of disease, and (iv) immunological sink, predominantly.[10] The anti-GBM antibodies in human disease are intrinsically heterogeneous and only some anti-GBM antibodies are pathogenic.[11] Atypical anti-GBM disease with putative “antibody negative” indicates that no single test can replace the accuracy of a good quality kidney biopsy.[12] As there was diffuse alveolar hemorrhage in our case, he received two sessions of PE. The decision of PE in the case of negative anti-GBM is challenging; however, associated severity of pulmonary manifestation favors the use of plasmapheresis.[10]
Our third case also presented as rapidly progressive renal failure with active urine sediments, which required dialysis support; however, her urine output was preserved till the end. This is again an unusual finding in crescentic GN. Alchi et al. studied 43 anti-GBM disease patients over 20 years and found that oligoanuria was the strongest predictor of both patient and renal outcomes. These patients had severe necrotizing GN in almost all glomeruli, and no patient in their study with oligoanuria recovered their renal function. Oligoanuria was also the strongest predictor of mortality.[4] This indicates that urine output in anti-GBM disease has significant prognostic value. There is consensus in the literature about the importance of early diagnosis and treatment before oligoanuria or the need to start dialysis in anti-GBM disease.[13,14] Our case also recovered her renal function; she is off dialysis and has serum creatinine of 1.8 mg/dL at 3 months of follow-up.
In pregnancy, the choice of immunosuppression is also crucial in anti-GBM disease. Cyclophosphamide, considered in category D, has been tried in pregnant women despite potential risks. In a clinical situation, if anti-GBM disease presents in the first trimester, termination of pregnancy should be preferred along with the usual treatment. In utero exposure to cyclophosphamide increases the child’s long-term risk of malignancies, papillary thyroid cancer, and neuroblastoma.[15] Systemic corticosteroids are generally considered low risk for teratogenicity, increase the risk of gestational diabetes, and with historical links to cleft palate, although this association is debatable. The safety profile of PE during pregnancy appears comparable to the application of PE in non-pregnant patients, except for the increased risk of hypotension.[16]
CONCLUSION
Anti-GBM disease can complicate pregnancy and increase the risk for both mother and fetus. Placenta can act as sink for anti-GBM antibodies, its delivery can complicate the clinical condition. The false-negative serology for anti-GBM antibodies can occur and histopathology is most definitive for diagnosis of anti-GBM disease. Urine output at the time of presentation is important prognostic clinical parameter.
Acknowledgment
We are very thankful to Dr. Pooja Maheshwari for her contribution in renal biopsy.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Anti-glomerular basement membrane disease. Rheum Dis Clin North Am. 2018;44:651-73.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical management of pregnancy in women with goodpasture syndrome. Gynecol Obstet Invest. 2015;79:73-7.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-glomerular basement membrane disease. Clin J Am Soc Nephrol. 2017;12:1162-72.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of renal and patient outcomes in anti-GBM disease: Clinicopathologic analysis of a two-centre cohort. Nephrol Dial Transplant. 2015;30:814-21.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal, pregnancy and fetal outcomes in de novo anti-glomerular basement membrane antibody disease in pregnancy: A systematic review. Clin Kidney J. 2014;7:450-6.
- [CrossRef] [PubMed] [Google Scholar]
- Goodpasture syndrome in a pregnant woman. Obstet Gynecol. 2005;106:1196-9.
- [CrossRef] [PubMed] [Google Scholar]
- Goodpasture syndrome in pregnancy without renal involvement: A case report. Casp J Intern Med. 2022;13:442-6.
- [Google Scholar]
- Reactivation of goodpasture disease during the third trimester of pregnancy: A case report. J Reprod Med. 2015;60:449-51.
- [Google Scholar]
- Atypical anti-glomerular basement membrane disease: Lessons learned. Clin Kidney J. 2016;9:653-6.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular architecture of the goodpasture autoantigen in anti-GBM nephritis. N Engl J Med. 2010;363:343-54.
- [CrossRef] [PubMed] [Google Scholar]
- Antibody-negative Goodpasture's disease. NDT Plus. 2010;3:253-6.
- [CrossRef] [PubMed] [Google Scholar]
- Course and prognosis of anti-basement membrane antibody (anti-BM-Ab)-mediated disease: Report of 35 cases. Nephrol Dial Transplant. 1994;9:372-6.
- [Google Scholar]
- Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med. 2001;134:1033-42.
- [CrossRef] [PubMed] [Google Scholar]
- Teratogenicity and carcinogenicity in a twin exposed in utero to cyclophosphamide. Teratog Carcinog Mutagen. 1993;13:139-43.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic plasma exchange in pregnancy: A literature review. Eur J Obstet Gynecol Reprod Biol. 2021;260:29-36.
- [CrossRef] [PubMed] [Google Scholar]







