Translate this page into:
Growth-associated protein-43, synapsin 1, and carbonic anhydrase 7 as biomarkers for seizures
*Corresponding author: Sridhar Amalakanti, Department of General Medicine, All India Institute of Medical Sciences (AIIMS), Mangalagiri, Andhra Pradesh, India. iamimenotu@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Amalakanti S, Arepalli K, Baranasi K, Jillella J. Growth-associated protein-43, synapsin 1, and carbonic anhydrase 7 as biomarkers for seizures. Indian J Med Sci. doi: 10.25259/IJMS_244_2023
Abstract
Objectives:
The objective of this study was to evaluate the utility of serum biomarkers for differentiating seizures from non-seizure paroxysmal episodes.
Materials and Methods:
We conducted a case–control study with 85 patients with confirmed seizure activity and 89 patients presenting with non-seizure paroxysmal events (controls). Serum growth-associated protein-43 (GAP-43), synapsin 1, and carbonic anhydrase 7 (CA 7) levels were measured using enzyme-linked immunosorbent assay.
Results:
Mean GAP-43 levels were higher in the seizure group (5.23 ng/mL) compared to controls (2.3 ng/mL) (P < 0.001). Mean synapsin 1 and CA 7 levels were elevated in seizure cases (8.8 μg/mL and 11.49 μg/mL) versus controls (5.24 μg/mL and 6.73 μg/mL) (both P < 0.001).
Conclusion:
Serum biomarkers GAP-43, synapsin 1, and CA 7 hold promise as rapid diagnostic tests to differentiate seizures from other paroxysmal neurological events. If validated clinically, these markers could aid time-sensitive diagnostic and therapeutic decision-making for patients.
Keywords
Seizures
Biomarkers
Growth-associated protein-43 protein
Synapsins
Carbonic anhydrases
Diagnosis
INTRODUCTION
Seizure is a sudden excessive discharge in the brain. This manifests as a paroxysmal behavioral abnormality in the patient. Patients with a tendency to get seizures are diagnosed with epilepsy. However, the diagnosis of seizures is challenging as they can occur unexpectedly, and their manifestations may not be accurately described by onlookers. Sometimes, the event is not visualized by any person. Moreover, some of the times, it is difficult even for an experienced clinician to confidently name an event as a seizure.[1]
The electroencephalogram (EEG) is employed to record the abnormal excessive discharges. However, many times, there may not be a signature of the seizure. There is also a class of seizures called non-convulsive seizures in which there are no visible behavioral changes. These are difficult to diagnose.[2] Thus, at present, the diagnosis of seizures, epilepsy, and seizure such as events or pseudo-seizures is far from precise.
The previous studies have proposed biomarkers for seizures such as miRNAs[3,4] and proteins[5,6] but none have been useful in clinical situations. Hence, a biomarker for seizure is very much needed.
Carbonic anhydrase 7 (CA 7),[7] a neuromodulator, synapsin-1 a synaptogenesis and synaptic plasticity modulator[8] and growth-associated protein-43 (GAP-43), a synapse growth potentiator[9] are associated with epilepsy in various studies. These promising proteins need to be assessed in clinically relevant situations.
CAs affect the pH transients in intra- and extracellular compartments. They influence the function of membrane proteins involved in neuronal signaling. CA 7 is present in the human cortex and hippocampus. It is effective in promoting GABAergic excitation and is associated with seizure disorders.[7]
Synapsins are a family of proteins expressed in the nervous system. They regulate neurite outgrowth, synaptic development, function, and plasticity through the regulation of the number of synaptic vesicles. Synapsin 1 (SYN1) isoform of synapsin has been independently associated with epilepsy.[8]
GAP-43 is a marker of newly formed synapses. Evidence suggests that GAP-43 plays an important role in epileptogenesis-induced synaptic plasticity in neural networks.[9]
The study aimed to assess the levels of these proteins in patients with seizures and other paroxysmal events. By measuring the levels of these proteins, clinicians can potentially differentiate seizures from other paroxysmal events, leading to more accurate diagnosis and treatment.
MATERIALS AND METHODS
This study is an observational study conducted from January 2021 to March 2023, with approval from the Institutional Medical Ethics Committee at a tertiary Medical School and Hospital. The sample size of 77 cases and 77 controls was calculated based on previous studies, with a power of 80% and a 95% confidence interval to detect a difference of at least 3 ng/ mL of GAP-43 or CA 7. An additional 10% of subjects were added to cater to dropouts. The study included 85 patients with a recorded generalized seizure event not on antiepileptic drugs and 89 controls between 18 and 60 years of age who provided written informed consent. Patients with associated stroke, migraine, or trauma were excluded from the study. One blood sample was collected within 24 h of the seizure/event.
Peripheral blood venous samples were collected from each participant (3 mL), clotted for 30 min at 37°C, and centrifuged for 15 min at 3,500 rpm. The separated sera were stored at 80°C until biochemical analysis. Serum human CA 7, human synapsin-1, and GAP-43 assays were measured using commercially available enzyme-linked immunosorbent assay kits supplied by Abbexa LTD, Cambridge, UK, Catalog No. abx386236, MyBioSource. com, San Diego, USA, Catalog No. MBS9714077 and United States Biological, Salem, USA, Catalog No. 383001, respectively, according to the manufacturer’s protocol.
The data were analyzed using the Statistical Package for the Social Sciences, (version 22.0, Chicago, IL). Quantitative data were represented by mean and standard deviation (SD) and analyzed by student t-test. Qualitative data were represented by proportions and analyzed by Chi-square test. Receiver operating characteristic (ROC) curves were plotted with optimum specificity to diagnose seizures. The tests were considered statistically significant if P < 0.05.
RESULTS
The study compared patients with generalized tonic-clonic seizures to other ictal events. In our study, the mean age (SD) of the cases (36.95 [10.12]) and controls (34.51 [11.59]) was similar (P = 0.06). Male-to-female ratio in cases (47:38) versus controls (45:44) was similar (P = 0.5). MRI of the brain was normal in all the subjects. All the patients had generalized tonic-clonic seizures. In the EEG recording, 78.7% of seizure patients showed a normal record, 8% showed generalized spike and wave discharges, 8.6% showed spike wave discharges, 2.9% showed slow waves, and 1.1% showed sharp waves. All the patients belonged to the seizure of unknown etiology category of ILAE classification. Among the controls, the most common diagnoses were acute gastroenteritis (15.73%) and anemia (14.61%). The most common ictus type was loss of consciousness (47.19%) and syncope (39.33%).
The mean GAP-43 levels were significantly higher in patients with seizures (5.23 ng/mL [1.5]) than in controls (2.3 ng/mL [0.83]) (P < 0.001). Synapsin-1 levels were significantly higher in patients with seizures (8.8 μg/mL [2.27]) than in controls (5.24 [1.7]) (P < 0.001). The mean CA 7 levels were significantly higher in patients with seizures (11.49 [3.06]) than in controls (6.73 [1.96]) (P < 0.001) [Figure 1].
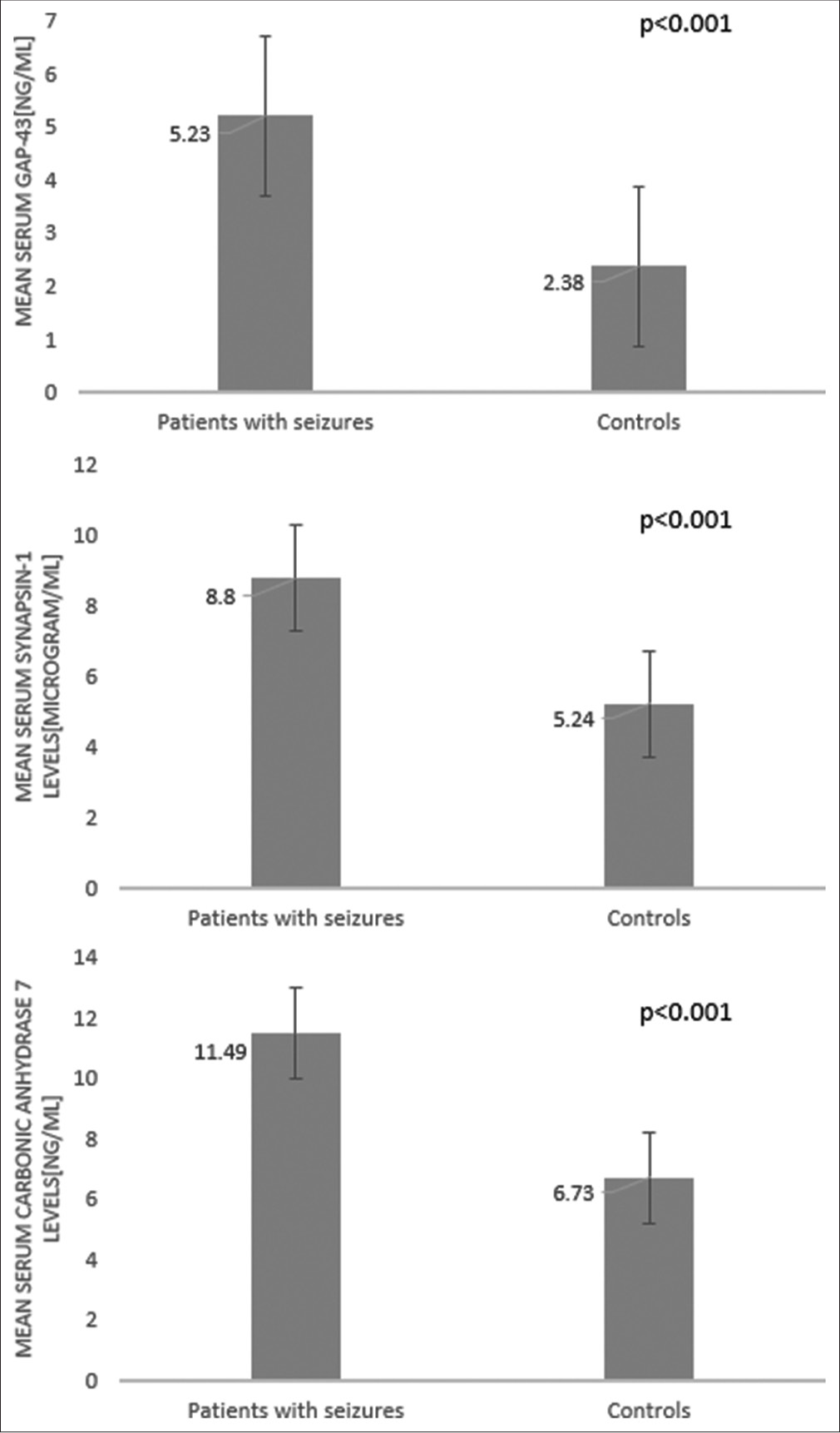
- The mean growth-associated protein-43 (ng/mL), synapsin-1 levels (µg/mL), and carbonic anhydrase 7 levels (ng/mL) were higher in patients with seizures than in controls, were higher in patients with seizures than in controls, and were higher in patients with seizures than in controls. GAP: Growth Associated Protein-43.
The ROC for GAP-43 showed a 0.96 area under curve. At a cutoff level of 3.57 ng/mL of GAP-43, the specificity was 100% and sensitivity 85%. The ROC for synapsin-1 showed a 0.89 area under curve. At a cutoff level of 7 ng/mL of synapsin-1, the specificity was 78% and sensitivity 75%. The ROC for CA 7 showed a 0.90 area under curve. At a cutoff level of 9.25 ng/mL of CA 7, the specificity was 100% and sensitivity 81% [Figure 2].
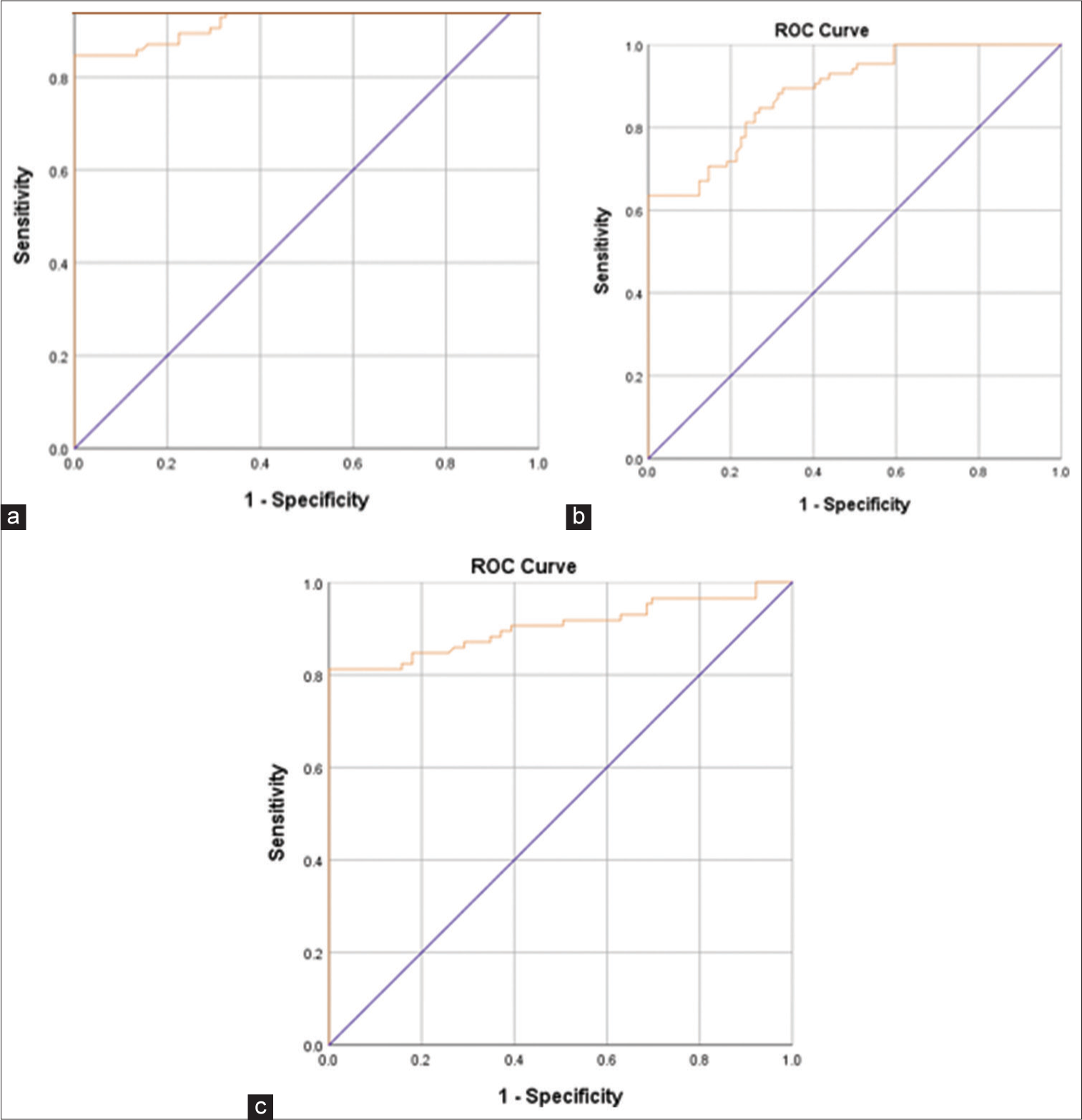
- The receiver operating characteristic (ROC) for (a) growth associated protein-43, (b) synapsin-1, and (c) carbonic anhydrase 7.
DISCUSSION
Our study investigated the levels of three proteins – GAP-43, synapsin-1, and CA 7 in the serum of seizure patients and non-seizure paroxysmal events. Our results showed that the levels of these proteins were significantly higher in patients with seizures when compared to other ictal events.
GAP-43 is an intracellular protein located at branching points, growth cones, and axon terminals involved in axonal elongation, neurotransmitter release, and synaptic vesicle recycling. It is activated through phosphorylation at ser41 through protein kinase C. GAP-43 is upregulated following exposure to excitatory neurotransmitters like glutamate.[10] Excitatory neurotransmitter release and synaptic vesicle modulation are important steps in seizures and epileptogenesis. GAP-43 is an important protein in this regard. Preclinical studies showed that seizures were positively correlated with higher GAP-43.[10] In addition to our study, previous studies performed on childhood epilepsy[9] and febrile seizures[11] found higher levels of GAP-43 levels in the subjects. It could be a reliable biomarker for seizures in clinical settings. At a cutoff level of 3.57 ng/mL of GAP-43, the specificity was 100% and sensitivity 85%. The test can exclude the seizure other paroxysmal events in making the diagnosis.
Synapsin 1 is a neuron-specific phosphoprotein regulating neurotransmitter release. Synapsin 1 contains an amphipathic lipid sensing motif that, by sensing membrane curvature, facilitates association with synaptic vesicles. It facilitates the recruitment of a reserve pool of synaptic vesicles during high neuronal activity.[12] The excessive neuronal activity during seizures suggests an important role for synapsin 1 and its detection in the serum corresponds to the pathophysiology. Together with its mutations associated with epilepsy[8] and our findings of higher synapsin 1 levels in cases than in controls, it also can be an important biomarker in seizures. At a cutoff level of 7 ng/mL of synapsin-1, the specificity was 78% and sensitivity 75%. Synapsin 1 may be used in conjunction with other biomarkers in making the diagnosis of seizures in doubtful cases.
CA 7 is expressed in the cortex, hippocampus, and thalamus regions within the mammalian brain.[13] It is involved in the mechanism of pH regulation which is essential in the modulation of various N-Methyl-d-aspartate, cation, and ligand-gated channels.[14] Seizures themselves are accompanied by alterations in pH. CA 7 also modulates excitatory GABA-mediated nerve impulses. Our study found significantly higher CA 7 levels in cases than in controls. Moreover, CA inhibitors have been useful in seizure therapy. Thus, the molecule is an essential mediator in seizure pathophysiology. Put together it is a potential biomarker for seizures. At a cutoff level of 9.25 ng/mL of CA 7, the specificity was 100% and sensitivity 81%. This serum level can thus help exclude other diagnoses.
However, we acknowledge that our study has some limitations, including its small sample size and the exclusion of seizures other than generalized tonic-clonic seizures and patients on AEDs. However, we show that GAP-43, synapsin 1, and CA 7 can be used to differentiate seizures from other paroxysmal events. Our study suggests that these proteins could potentially serve as biomarkers for seizures, with specific cutoff levels providing high sensitivity and specificity. Since many of the ictal events are sudden and without witnesses, these simple tests are beneficial to the public. Our study highlights the potential utility of these three proteins as simple and portable diagnostic tools, particularly in resource-limited settings where sophisticated investigations may not be available.
CONCLUSION
Overall, this work presents compelling data supporting the potential utility of serum levels of GAP-43, synapsin 1, and CA VII as biomarkers for distinguishing seizures from other paroxysmal occurrences. The results demonstrated markedly elevated levels of these proteins in individuals with seizures in comparison to the control group, exhibiting a high degree of specificity and sensitivity at certain threshold values. Although more verification in larger clinical environments is necessary, these biomarkers have the potential to assist in prompt and precise diagnosis, resulting in timely treatment choices for patients exhibiting seizure-like episodes.
Authorship contributions
SA: Conceptualization. SA, KVRA, KB, and JJP: Methodology. SA, KVRA, KB, and JJP: Formal Analysis. SA, KVRA, KB, and JJP: Investigation. SA: Original Draft Preparation. Review and Editing: SA, KVRA, KB, and JJP.
Ethical approval
The research/study approved by the Institutional Review Board at Great Eastern Medical School and Hospital, number 85/IEC/GEMS/2020, dated December 01, 2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Diagnostic challenges in epilepsy: Seizure under-reporting and seizure detection. Lancet Neurol. 2018;17:279-88.
- [CrossRef] [PubMed] [Google Scholar]
- Nonconvulsive status epilepticus: A diagnostic and therapeutic challenge in the intensive care setting. Ther Adv Neurol Disord. 2011;4:169-81.
- [CrossRef] [PubMed] [Google Scholar]
- MicroRNA-134 plasma levels before and after treatment with valproic acid for epilepsy patients. Oncotarget. 2017;8:72748-4.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of serum miRNAs differentially expressed in human epilepsy at seizure onset and post-seizure. Mol Med Rep. 2016;14:5318-24.
- [CrossRef] [PubMed] [Google Scholar]
- Time-dependent changes in the serum levels of prolactin, nesfatin-1 and ghrelin as a marker of epileptic attacks young male patients. Peptides. 2011;32:1276-80.
- [CrossRef] [PubMed] [Google Scholar]
- Tetranectin is a potential biomarker in cerebrospinal fluid and serum of patients with epilepsy. Clin Chim Acta. 2010;411:581-3.
- [CrossRef] [PubMed] [Google Scholar]
- Neuronal carbonic anhydrase VII provides GABAergic excitatory drive to exacerbate febrile seizures. EMBO J. 2013;32:2275-86.
- [CrossRef] [PubMed] [Google Scholar]
- The different clinical facets of SYN1-related neurodevelopmental disorders. Front Cell Dev Biol. 2022;10:1019715.
- [CrossRef] [PubMed] [Google Scholar]
- Serum levels of growth-associated protein-43 and neurotrophin-3 in childhood epilepsy and their relation to zinc levels. Biol Trace Elem Res. 2023;201:689-97.
- [CrossRef] [PubMed] [Google Scholar]
- Growth associated protein 43 (GAP-43) as a novel target for the diagnosis, treatment and prevention of epileptogenesis. Sci Rep. 2017;7:17702.
- [CrossRef] [PubMed] [Google Scholar]
- Biochemical assessments of neurotrophin-3 and zinc involvement in the pathophysiology of pediatric febrile seizures: Biochemical markers in febrile seizures. Biol Trace Elem Res. 2022;200:2614-9.
- [CrossRef] [PubMed] [Google Scholar]
- Synapsin I senses membrane curvature by an amphipathic lipid packing sensor motif. J Neurosci. 2011;31:18149-54.
- [CrossRef] [PubMed] [Google Scholar]
- Structure-based screening for the discovery of new carbonic anhydrase VII inhibitors. Eur J Med Chem. 2014;71:105-11.
- [CrossRef] [PubMed] [Google Scholar]
- Carbonic anhydrase inhibitors and epilepsy: State of the art and future perspectives. Molecules. 2021;26:6380.
- [CrossRef] [PubMed] [Google Scholar]






