Translate this page into:
Navigating prognostic uncertainty in non-muscle invasive urothelial bladder cancer with focal squamous differentiation
*Corresponding author: Ankur Mittal, Department of Urology, AIIMS, Rishikesh, Uttarakhand, India. departmenturologyaiimsrksh@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Shubhankar G, Mittal A, Panwar VK, Tosh JM, Mandal AK. Navigating prognostic uncertainty in non-muscle invasive urothelial bladder cancer with focal squamous differentiation. Indian J Med Sci. doi: 10.25259/IJMS_277_2024
Abstract
Objectives
Transitional cell carcinoma (TCC), or urothelial carcinoma, represents the predominant histological type of bladder cancer. While the majority of TCC cases are characterized by conventional urothelial features, a significant subset exhibits variant histologies, such as focal squamous differentiation (SD). This variant is clinically noteworthy, given its association with more aggressive disease behavior and potential resistance to standard therapies, particularly intravesical Bacillus Calmette–Guérin (BCG) therapy. It raises critical challenges in prognosis and treatment.
Materials and Methods
This single-center prospective cohort study enrolled patients with histopathologically confirmed TCC of the bladder, with or without focal SD, following transurethral resection of bladder tumor. Participants, devoid of deep muscle invasion and residual growth, received intravesical BCG therapy consisting of six weekly induction doses followed by 12 months of maintenance therapy. Outcomes were assessed through regular follow-up, utilizing clinical evaluations, imaging, urine cytology, and cystoscopy. The primary endpoints included recurrence-free survival (RFS), progression-free survival (PFS), and disease-specific survival (DSS).
Results
Of the 131 patients evaluated, 35 exhibited focal SD. Statistically significant differences were observed between TCC with and without SD groups, with the former demonstrating poorer PFS (68.6% vs. 82.6%) and DSS (88.6% vs. 98%) after 1 year. However, RFS rates were comparable. Subgroup analysis revealed no significant differences in outcomes based on the extent of SD (>50% vs. <50%).
Conclusion
Focal SD within TCC of the bladder portends a worse prognosis, particularly in terms of PFS and DSS, underscoring the necessity for tailored therapeutic approaches.
Keywords
Bacillus Calmette–Guérin therapy
Focal squamous differentiation
Intravesical therapy
Non-muscle invasive bladder cancer
Variant histology
INTRODUCTION
Transitional cell carcinoma (TCC) of the bladder, also referred to as urothelial carcinoma, stands as the most prevalent histological subtype of bladder cancer. While the majority of TCC cases are typified by conventional urothelial features, a noteworthy subset of these tumors exhibits variant histologies, including focal squamous differentiation (SD). This variant, observed in approximately 20% of bladder cancer cases, is of particular concern due to its association with more aggressive clinical behavior. Such characteristics inevitably raise significant challenges in prognosis and therapeutic management.[1]
Focal SD within TCC is defined as the presence of SD involving more than 10% of the tumor area.[1,2] The squamous cells are interspersed within the urothelial tumor, typically identified through meticulous histopathological examination. The clinical relevance of this variant lies in its potential to markedly influence the disease course, response to treatment, and overall survival outcomes. Indeed, the presence of SD has been correlated with a diminished sensitivity to intravesical Bacillus Calmette–Guérin (BCG) therapy, which remains a cornerstone in the management of non-muscle invasive bladder cancer (NMIBC). The reduced efficacy of BCG in these cases may lead to higher recurrence rates and an increased propensity for progression to muscle-invasive bladder cancer (MIBC), thus complicating the therapeutic landscape and prognostic assessments for affected patients.[3-5]
Despite the clinical significance of focal SD, the existing literature on its impact on TCC remains relatively sparse. Studies by Bilski et al. and Chade et al. have provided valuable insights into the potential prognostic implications of this histological variant, yet the evidence base is constrained by small sample sizes and the predominantly retrospective nature of research to date.[3,6] Moreover, heterogeneity in the definition and reporting of SD across studies further obfuscates the ability to draw definitive conclusions. Consequently, there is a pressing need for robust, prospective research to elucidate the prognostic significance of focal SD and optimize therapeutic strategies for this challenging subset of bladder cancer patients. To address this gap, we conducted a prospective observational study among NMIBC patients receiving intravesical BCG therapy, aiming to determine whether variant histology bladder tumors exhibit differential outcomes, thereby refining our prognostic capabilities.
MATERIALS AND METHODS
It was a single-center prospective cohort study conducted at our institute between August 2018 and December 2023. Before commencement, approval was secured from the Institutional Ethics Committee (Approval No: AIIMS/IEC/21-2021), and informed consent was obtained from all participants. The study included patients with histopathologically confirmed TCC of the bladder with focal SD or those with TCC without focal SD, identified post-transurethral resection of bladder tumor (TURBT), without deep muscle invasion and no residual growth, who were scheduled for intravesical BCG therapy. Patients underwent restage TURBT before enrolment to ensure complete tumor clearance, particularly for those with multifocal tumors.
All the patients meeting the inclusion criteria received intravesical BCG therapy. The therapeutic regimen consisted of an initial induction phase with six weekly doses of intravesical BCG, followed by a maintenance phase entailing monthly administrations for a total duration of 12 months. Each BCG dose was set at 120 mg. Following instillation, patients were instructed to retain urine for 1 h and change positions every 15 min to optimize the therapeutic contact of BCG with the urothelium. In cases where tolerance issues arose, the dose was reduced to 80 mg, with subsequent evaluation of response. The 1-h retention time follows institutional protocol; however, the standard recommendation in literature varies between 1 h and 2 h.[7]
Patients were monitored through regular follow-up appointments every 3 months. Assessments included clinical evaluations, abdominal ultrasound, urine cytology, and cystoscopy.
The impact of the presence of focal SD on various prognostic factors – including recurrence-free survival (RFS), disease-free survival (DFS), progression-free survival (PFS), and overall survival – was analyzed and compared with the patients of TCC without SD after 1 year of treatment. Statistical analysis was performed using the Statistical Package for the Social Sciences version 28. Proportions were compared using the Chi-square test, and means were assessed with the Student’s t-test. The significance threshold was set at P = 0.05, corresponding to a 95% confidence interval.
Kaplan–Meier analysis was employed to determine RFS, PFS, and DFS rates to evaluate survival outcomes. This comprehensive methodology ensured a rigorous assessment of the therapeutic impact of BCG in the context of focal SD in NMIBC, providing valuable insights into the efficacy and prognostic implications of this variant histology.
RESULTS
Out of the final cohort of 131 patients, 35 patients (23%) had TCC with focal SD, while 96 had TCC without SD. The demographic characteristics of the two groups, including age, gender, smoking history, and comorbidities, were comparable and did not show statistically significant differences [Table 1]. Nineteen patients (16% of the total cohort) experienced significant side effects that necessitated a reduction in the BCG dose from 120 mg to 80 mg. Among these, 16 patients completed the therapy at the lower dose, whereas BCG therapy was discontinued in 3 patients due to persistent intolerance, even at the reduced dosage. Among the 19 patients who required BCG dose reduction, 5 developed disease progression, 6 had recurrence, and 3 exhibited stage migration.
| Features | TCC with SD | TCC without SD | P-value |
|---|---|---|---|
| No. of cases | 35 | 96 | |
| Age (years) | 61±12 | 63±11 | 0.37 |
| Sex (Male: Female) | 6:1 | 5.5:1 | 0.77 |
| Smokers | 28 | 79 | 0.78 |
| Grade | |||
| Low | 4 | 28 | 0.06 |
| High | 31 | 68 | |
| Size of tumor (cm) (Mean±Standard deviation) | 3±1.5 cm | 3.5±1 cm | 0.07 |
| Multifocal tumors | 29 | 80 | 1.0 |
TCC: Transitional cell carcinoma, SD: Squamous differentiation
Within the TCC without SD group, 68 patients (67%) were classified as having T1 high-grade disease, and 28 patients (33%) had T1 low-grade disease, all of whom fell into the high-risk category. Following BCG therapy as per the established protocol, the median follow-up duration was 12 months. During this period, 84.9% (n = 81) of patients remained recurrence-free. 82.6% (n = 79) of the patients did not progress to MIBC and 98% (n = 94) were alive without bladder cancer-related mortality. In the TCC with SD group, following 12-month median follow-up, 71.4% (n = 25) remained recurrence-free, a marginally lower rate than the non-SD group, but not statistically significant (P = 0.52). 68.6% (n = 24) of patients did not progress to MIBC, significantly lower than the non-SD group (P = 0.049) and 88.6% (n = 31) were alive without bladder cancer-related mortality, which was significantly poorer compared to the non-SD group (P = 0.007) [Figures 1-3].
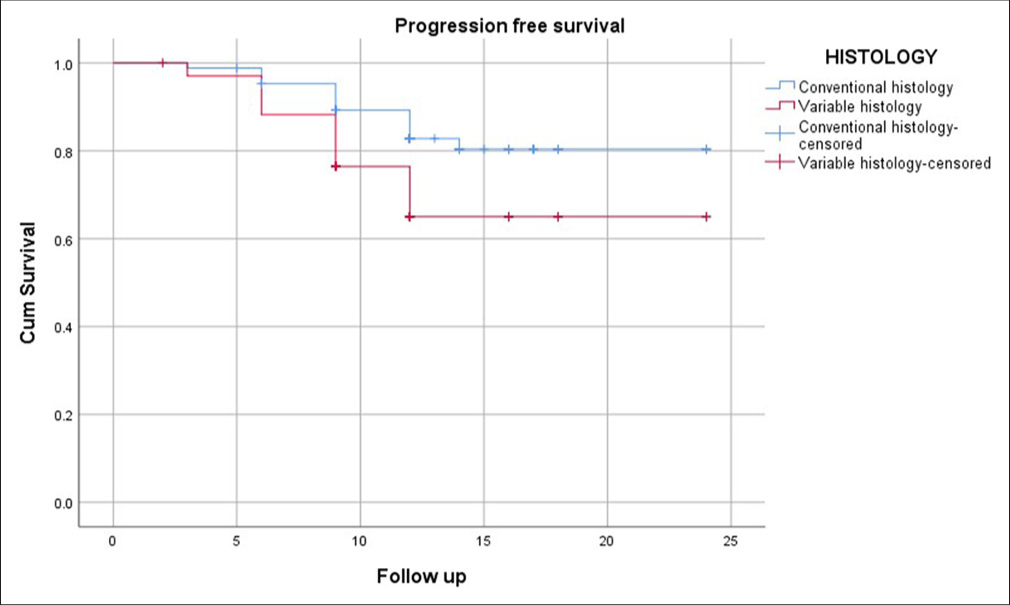
- Kaplan–Meier curve showing progression-free survival 1 year (Blue line – TCC without SD; Red Line – TCC with SD). TCC: Transitional cell carcinoma, SD: Squamous differentiation.
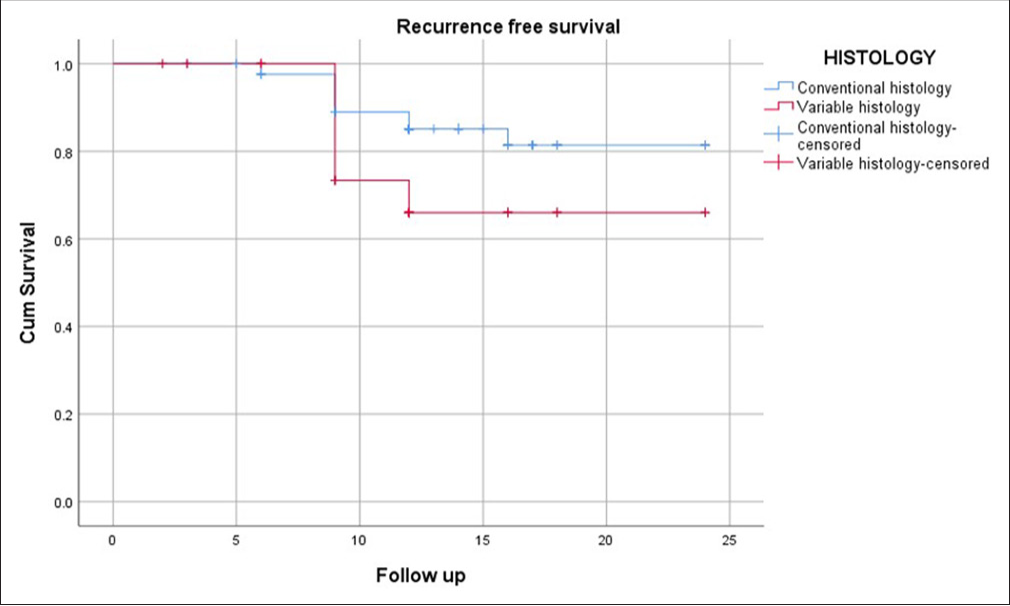
- Kaplan–Meier curve showing recurrence-free survival 1 year (Blue line – TCC without SD; Red Line – TCC with SD). TCC: Transitional cell carcinoma, SD: Squamous differentiation.
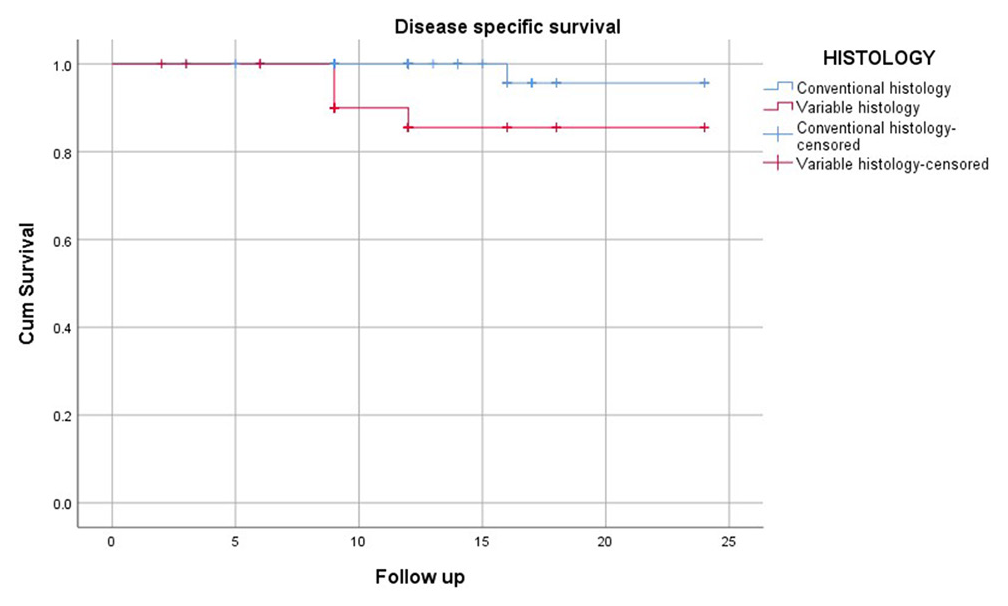
- Kaplan–Meier curve showing disease-free survival 1 year (Blue line – TCC without SD; Red Line – TCC with SD). TCC: Transitional cell carcinoma, SD: Squamous differentiation.
Kaplan–Meier survival curves further illustrated the disparity in survival outcomes between patients with and without SD. The curves demonstrated a steeper decline in PFS and DSS among patients with SD, particularly within the 1st year of follow-up. Comparable RFS curves between the SD and non-SD groups highlighted that recurrence rates were similar, regardless of variant histology.
A subgroup analysis within the SD group was also done to evaluate the potential impact of the extent of SD. Patients with TCC and focal SD were stratified into two subgroups based on histological findings: SD >50% (n = 12) and SD <50% (n = 23). In the SD >50% subgroup, the mean RFS was 11 ± 0.57 months, PFS 10.1 ± 0.9 months, and DSS 11.5 ± 0.45 months. Among the SD <50% subgroup, the mean RFS was 19 ± 1.5 months, PFS 18.4 ± 1.6 months, and DSS 22.3 ± 1.1 months. Although patients with SD <50% appeared to fare better in terms of survival metrics, the differences between the two subgroups were not statistically significant for RFS (P = 0.935), PFS (P = 0.728), or DSS (P = 0.696).
DISCUSSION
The present study aimed to elucidate the prognostic implications of focal SD in NMIBC treated with intravesical BCG therapy. Our findings underscore the complexity of managing TCC with SD, particularly in the context of NMIBC, where BCG therapy remains the cornerstone of treatment.
The data from our cohort revealed that patients with TCC featuring focal SD exhibited significantly poorer PFS and disease-specific survival (DSS) rates compared to those with TCC lacking this histological variant. Specifically, the PFS and DSS in the SD group were 68.6% and 88.6%, respectively, at 1 year, compared to 82.6% and 98% in the non-SD group. These findings align with previous studies, such as those by Soave et al. and Bilski et al., which also reported a worse prognosis in patients with SD.[1,3] Soave et al. highlighted that SD is a critical prognostic factor in MIBC, with significant implications for survival outcomes.[1]
Our study further corroborates the observations made by Chade et al., who reported that the presence of SD in bladder cancer is associated with a more aggressive disease course. Although Chade’s study focused on radical cystectomy outcomes in MIBC, the parallels in our NMIBC cohort suggest that the aggressive nature of SD is not limited to invasive disease but extends to the non-muscle invasive stage as well.[6,8]
Interestingly, the RFS rates between the SD and non-SD groups did not show a statistically significant difference, indicating that while focal SD may not necessarily increase the likelihood of early recurrence post-BCG therapy, it does appear to predispose patients to more severe disease progression and reduced survival. This finding suggests that RFS alone may not be a sufficient metric for evaluating prognosis in NMIBC patients with variant histology, particularly those with SD.
Subgroup analysis of our patients with TCC with SD revealed no significant differences in outcomes based on the extent of SD (>50% vs. <50%). This suggests that the mere presence of SD, regardless of its extent, may be sufficient to confer a worse prognosis. However, the lack of statistical significance in these comparisons could be attributed to the relatively small sample size, echoing the limitations highlighted by previous studies such as Shapur et al.[9]
The implications of these findings are multifaceted. First, they suggest that NMIBC patients with focal SD may benefit from more aggressive therapeutic strategies, possibly including early cystectomy, as proposed by Kamat et al., particularly in cases where BCG therapy fails to achieve adequate disease control.[10] In addition, these results underscore the necessity for continued research into alternative and adjunctive treatments that could potentially enhance the efficacy of BCG in this challenging patient subset.
Our study reinforces the notion that focal SD in NMIBC is a significant prognostic factor, warranting careful consideration in therapeutic decision-making. The findings advocate for a more nuanced approach to the management of TCC with SD, recognizing the limitations of current treatment modalities and the need for further exploration of tailored therapeutic strategies. However, the study was single-centered and the sample size was small with a median follow-up of 1 year till now. Hence, larger sample size studies with longer follow-ups might be needed for further exploration in this context.
CONCLUSION
This study illuminates the prognostic challenges posed by focal SD in NMIBC treated with intravesical BCG therapy. Our findings reveal that patients with TCC featuring focal SD exhibit significantly poorer PFS and DSS compared to those without SD, underscoring the aggressive nature of this histological variant. Notably, subgroup analysis indicated that the extent of SD (>50% vs. <50%) did not significantly impact outcomes, suggesting that even minimal SD can portend a worse prognosis. These results advocate for heightened vigilance and potentially more aggressive therapeutic approaches in managing TCC with SD. Given the limitations of this study, including its single-center design and modest sample size, further research with larger cohorts and longer follow-ups is essential. Until then, patients with TCC with SD should be closely monitored for potential disease progression.
Ethical approval
The research/study approved by the Institutional Review Board at All India Institute of Medical Sciences, Rishikesh, number AIIMS/IEC/21-2021, dated 17th August 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Prognostic role of squamous differentiation in muscle-invasive bladder cancer. J Urol. 2015;193:1206-11.
- [Google Scholar]
- Impact of squamous differentiation in bladder cancer treated with BCG. J Clin Oncol. 2017;35:457.
- [Google Scholar]
- Bladder cancer variant histology: Clinical implications and treatment strategies. Urol Oncol. 2018;36:93-9.
- [Google Scholar]
- The role of histological variants in bladder cancer prognosis. Cancer Med. 2021;10:1623-30.
- [Google Scholar]
- Outcomes of radical cystectomy for bladder cancer with squamous differentiation. Int Braz J Urol. 2010;36:48-54.
- [Google Scholar]
- Evaluating treatment response in NMIBC with variant histology. Clin Genitourin Cancer. 2022;20:e115-23.
- [Google Scholar]
- Is radical cystectomy mandatory in every patient with variant histology of bladder cancer? Rare Tumors. 2011;3:e22.
- [CrossRef] [PubMed] [Google Scholar]
- The case for early cystectomy in the treatment of nonmuscle invasive micropapillary bladder carcinoma. J Urol. 2006;175:881-5.
- [CrossRef] [PubMed] [Google Scholar]






