Translate this page into:
Prevalence of HLA-B*27 in the North Indian population: A single-center study
*Corresponding author: Vikash Chandra Mishra, Department of Molecular Genetics and Transplant Immunology, Chimera Transplant Research Foundation, New Delhi, India. vikashbiotech01@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mishra VC, Chandra D, Raina A, Pandey A, Raina V. Prevalence of HLA-B*27 in the North Indian population: A single-center study. Indian J Med Sci. doi: 10.25259/IJMS_131_2024
Abstract
Objectives:
Ankylosing spondylitis (AS) is a type of spondylitis in which the spine and other joints are largely affected. A strong association of HLA-B*27 with AS has been observed. However, the exact mechanism underlying this association and the pathogenesis of the disease remains unclear. As the diversity of HLA varies among populations, our objective was to investigate the prevalence of HLA-B*27 and its allelic variants in the North Indian population.
Materials and Methods:
The data of 7451 individuals were analyzed for the study and all these samples have HLA typed by sequence-based typing from an American Society for Histocompatibility and Immunogenetics (ASHI)-accredited laboratory.
Results:
Among the studied population, 304 individuals (4.08%) were found to have HLA-B* 27 allele. The most frequent subtypes of HLA-B*27 observed in the North Indian population were HLA-B*27: 05 (21.71%) and HLA-B*27: 07 (16.28%). A total of 97 HLA-B genotypes were identified among studied population.
Conclusion:
The higher frequency of HLA-B*27:05 suggests an idea that the North Indian population may have a higher risk of AS.
Keywords
Human leukocyte antigen B27
Human leukocyte antigen B27 frequency
North India
Ankylosing spondylitis
INTRODUCTION
Ankylosing spondylitis (AS) is a chronic immune-mediated inflammatory disease, part of the spondyloarthritis group, primarily affecting the sacroiliac joint and later the spine and large joints.[1] In South Asia, the incidence of AS ranges from 3.0 to 24.3/10,000 people, with a mean of 8.5/10,000, while in East Asia, it ranges between 11.0 and 37.1/10,000, with a mean of 25.5/10,000.[2-7] Hospital-based studies suggest a lower prevalence at 0.7/10,000.[8] AS is more common in men than women, with a 2:1 ratio, typically affecting people aged 20–40.[9,10] While the exact cause of AS is not fully understood, environmental factors (such as infections, exposure to heavy metals, stress, and cigarette smoking) and the human leukocyte antigen B27 (HLA-B*27) allele are considered major risk factors. However, the association between HLA-B27 and AS is still debated in rheumatology.[11]
Three primary theories attempt to explain the role of HLA-B*27 in AS. The “arthritogenic peptide hypothesis” suggests that HLA-B*27 mimics pathogens, triggering immune system activation and leading to AS.[12] Another theory proposes that mis-folded HLA-B*27 in macrophages causes endoplasmic reticulum stress and inflammation.[13] A third hypothesis involves the formation of HLA-B*27 homodimers, which stimulate natural killer cells and CD4+ T-cells, resulting in chronic inflammation.[14]
The HLA-B*27 allele is highly polymorphic, with 161 known subtypes. HLA-B27:05 is the most common and is closely associated with AS susceptibility.[15] Other associated subtypes include HLA-B*27:01, B*27:02, B*27:04, and B*27:07, while B*27:06 and B*27:09 are not significantly linked to AS.[16]
India’s diverse population, with its many tribes, castes, and religious groups practicing endogamy, has resulted in unique HLA gene complexes across communities.[17,18] Understanding the prevalence of HLA-B*27 in these populations, particularly in North India, is crucial for predicting the risk of AS and improving the quality of life for those affected.
MATERIALS AND METHODS
Study setting and demographics
The samples of 7451 healthy, normal individuals from North India, were examined for HLA-B typing in this study. All these individuals consented to be voluntary stem cell donors and registered themselves with a stem cell registry operating in North India. The sample collection and, further, HLA typing of all these individuals were done after taking informed consent. This study only included people aged between 18 and 55 years from Delhi, Punjab, Uttarakhand, Haryana, and Jammu and Kashmir. There were 5928 males and 1523 females in the group. The present study was performed to see the occurrence of HLA-B*27 in the North Indian population by estimating the allelic and genotypic frequency. The study was approved by the independent ethical review boards of the institute.
Sample collection and HLA typing
Blood samples (3 mL) were collected from each participant using ethylene diamine tetraacetic acid coated vials (BD Vacutainer®, USA). All these collected samples were further HLA typed by an accredited laboratory using the sequence-based typing method which covered Exon 2, 3, and 4 HLA-B locus.
Statistical analysis
HLA-B allele and genotypic frequencies were calculated by the direct counting method. For direct counting, a python script was created. The script calculates the frequency of an allele of interest depending on the number of times that it appears in the dataset.
RESULTS
A total of 304 individuals (4.08%) were identified with HLA-B*27 among the studied populations (n = 7451). Out of these 304 individuals, 239 (78.61%) were male, while the remaining 65 (21.38%) were female.
There were four allelic variants of HLA-B*27 (B*27:02, B*27:04, B*27:05, and B*27:07) identified among the 304 individuals. The most prevalent allelic variants of HLA-B*27 observed in our study population were HLA-B*27:05 (21.71%) and HLA-B*27:07 (16.28%).
The allele frequencies of all the identified allelic variants are depicted in Figure 1. In addition, 97 HLA-B* genotypes (allelic combinations) were observed within the studied population data, as illustrated in Figure 2. The most prevalent genotypic combinations identified were HLA-B*27:07–51:01 (4.60%), HLA-B*08:01–27:07 (4.27%), and HLA-B*27:05–40:06 (4.27%).
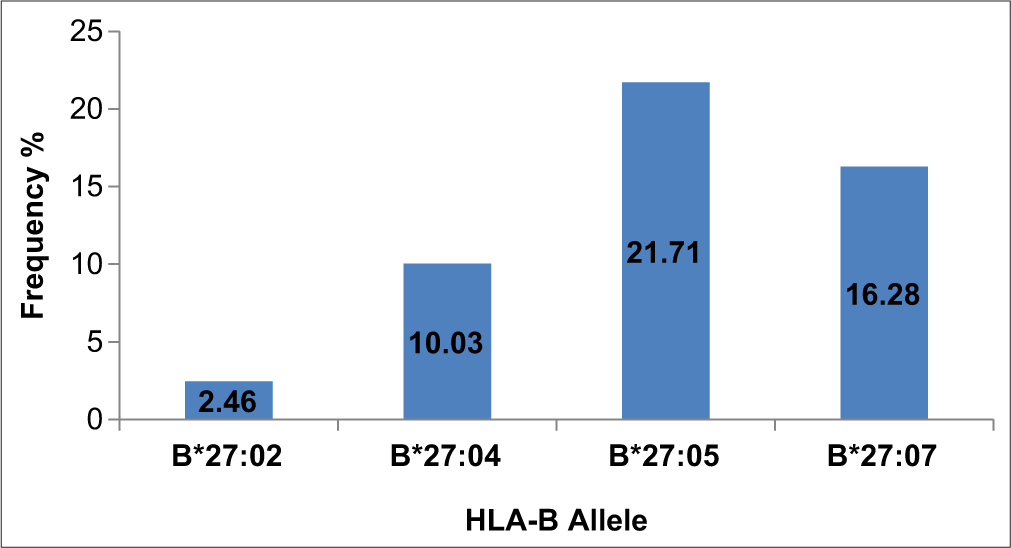
- HLA B27 allele frequency in the studied population group. HLA: Human leukocyte antigen.
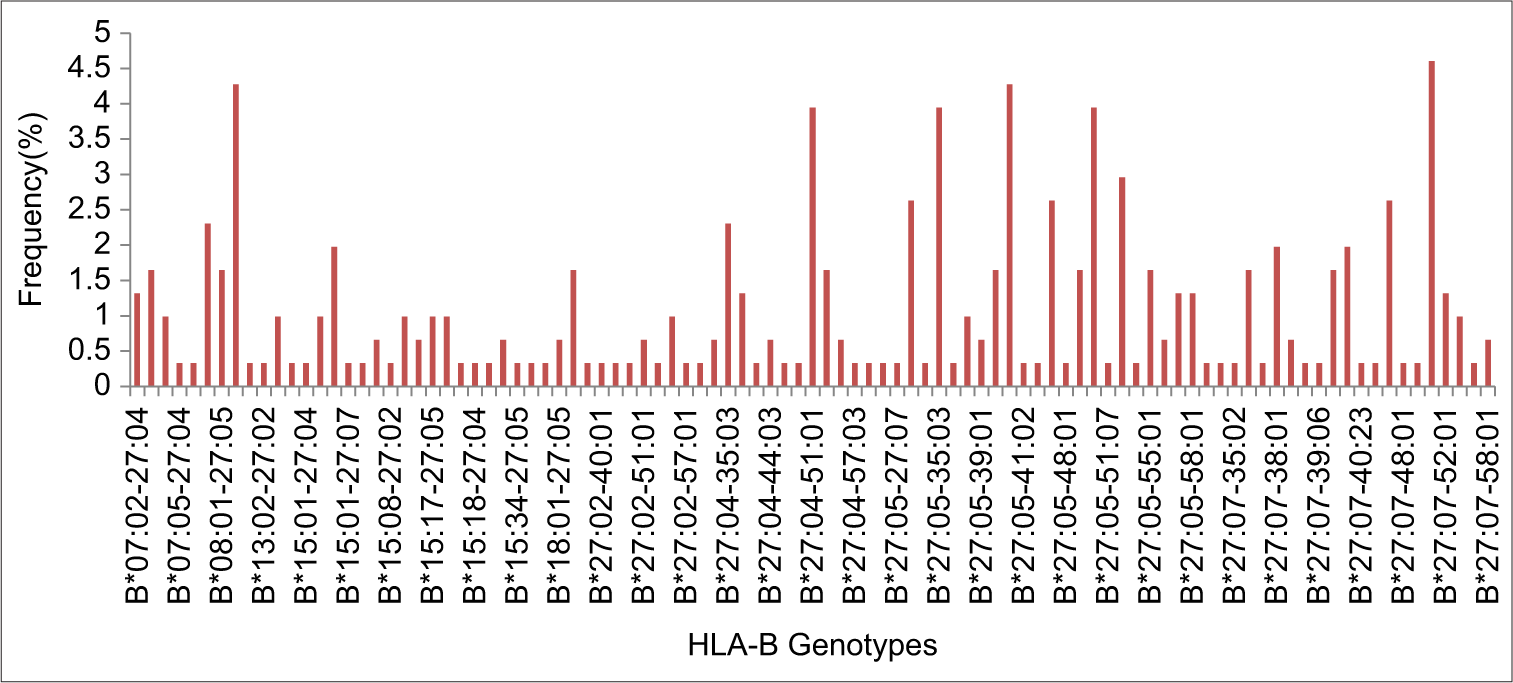
- Frequency of HLA B27 allelic combinations (genotypes) in the studied population group. HLA: Human leukocyte antigen.
DISCUSSION
The HLA complex encodes for the different classes of major histocompatibility complex proteins (Class I and II) that are responsible for the regulation of the immune system. The HLA complex is diverse, with many variations in the human population. There are specific patterns of these HLA variations among people from different geographical regions and even among individuals of different castes and religions. Not all variations of HLA-B*27 are linked to AS.[19,20] In this study, the most common variations of HLA-B*27 observed were HLA-B*27:05 (21.71%) and HLA-B*27:07 (16.28%).
Schlosstein et al. 1st time observed the relationship between the risk of AS and HLA-B*27 antigen.[21] The results of some studies showed that genetic predisposition of polymorphic HLA-B*27 allelic variants affects an individual’s susceptibility toward the development of AS while other results are contradictory.[22,23] At present, 90–95% of AS patients showed a positive test for HLA-B*27 allele.[5,24] In addition, a history of inflammatory backaches and a positive HLA-B*27 test are useful in making the diagnosis of AS. However, in the general population, a positive HLA-B*27 test that ranged between 6% and 8% only, does not confirm the disease and the majority of people having the HLA-B*27 will not develop AS, as well as, a negative HLA-B*27 test does not rule out the diagnosis of AS. As evidence, around 10–20% of individuals with confirmed AS found a negative test for the HLA-B*27 test.[25] These contradictory results show that diversity in the HLA gene is specific to the population as well as within the caste of a different place. Further, Shankarkumar observed that the frequency of HLA-B*27 was higher (11.76%) in the Western Indian group (Maratha) as compared (4.31%) to Indian tribal (Badagas).[26] In the last report of a community-oriented program for control of rheumatic disease, it was found that the prevalence of AS is more common in rural populations as compared to urban populations.[4] Based on these observations, HLA-B*27 should also be divided based on urban and rural groups for further use for the early screening of AS or aware the population who are at higher risk, and recommendations should be based on diet and other environmental factors (such as infections, exposure to heavy metals, stress, and cigarette smoking) as well as recommended physical activity or exercise.
HLA-B*27 positivity differs among the patients diagnosed with AS based on race and ethnicity. A study on Turkish population showed low prevalence of the HLA-B*27.[22,27,28] As per the published report, the most common HLA-B*27 allelic variants that are associated with the AS include HLA-B*27:05, HLA-B*27:04, and HLA-B*27:02 for Caucasians, Chinese (Asian), and Mediterranean populations, respectively.[15]
Thomas et al. reported the association of HLA-B*27:05 and B*27:04 with AS and the most prevalent allelic variant was B*27:05 (77.4%) in the South Indian population.[29] Another study from South India by Kavadichanda et al. identified, the HLA-B*27:04, 27:05, 27:07, and 27:02 association with AS and the among them HLA-B*27:04 (62.5%) was most prevalent allelic variant.[30] Whereas, in West India, HLA-B*27:02; 27:04; 27:05; and 27:07 were reported to be associated with AS with HLA-B*27:05 (67.9%) as the most prevalent allelic variants identified.[31] A study from North India by Srivastava et al. suggested an association between HLA-B*27:04, 27:05, 27:07, and 27:02 with AS .[32] Among these, HLA-B*27:05 (62.5%) was the most prevalent, followed by HLA-B*27:04 (36.2%) in the region. Similarly, in the present study, the most prevalent HLA-B allelic variants were HLA-B*27:05 (21.71%) and HLA-B27:07 (16.28%). A study in the late 90’s by Agrawal et al. did not find the presence of HLA-B*27 antigen in the North Indian population.[18] These contradictory results indicate that diversity in HLA genes is both population-specific and varies among castes across different regions. In addition, the techniques used for HLA typing (serology or molecular) and the sample size may also contribute to the presence or absence of these alleles. However, in the present scenario including the present study, the prevalence of HLA-B*27 is measurable which could help in the prevision of the likelihood of AS incidence. It is better to detect AS in its early stage because, in later stages, it involves severe complications such as anterior uveitis, psoriasis, and chronic inflammatory bowel disease, leading to cardiovascular or pulmonary complications. Therefore, prevision for the risk of AS is necessary. A strong association of HLA-B*27 with AS has been observed. However, the exact mechanism underlying this association and the pathogenesis of the disease remains unclear. Therefore, based on the frequency pattern of HLA-B*27 in a given population and caste, one can predict or diagnose an individual for the risk of AS.
CONCLUSION
In the present study, the North Indian population showed a higher frequency of HLA-B*27:05 and this suggests an idea that the people of this part may have a higher risk of AS. Further, systematic studies on large sample sizes investigating the combined effect of demographical as well as environmental risk factors associated with HLA-B*27 in the risk of developing AS are needed to be examined.
Ethical approval
The research/study approved by the Institutional Review Board at GENEBANDHU, number ECG006/2017, dated 27th December, 2017.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Role of HLA-B alleles and clinical presentation of b27 negative spondyloarthritis patients from Mumbai, Western India. Autoimmune Dis. 2014;2014:327315.
- [CrossRef] [Google Scholar]
- The prevalence of rheumatic diseases in a Filipino urban population: A WHO-ILAR COPCORD Study. World Health Organization. International League of Associations for Rheumatology. Community Oriented Programme for the Control of the Rheumatic Diseases. J Rheumatol. 1997;24:1814-9.
- [Google Scholar]
- Is there an urban-rural divide? Population surveys of rheumatic musculoskeletal disorders in the Pune region of India using the COPCORD Bhigwan model. J Rheumatol. 2009;36:614-22.
- [CrossRef] [Google Scholar]
- Global prevalence of ankylosing spondylitis. Rheumatology (Oxford). 2014;53:650-7.
- [CrossRef] [Google Scholar]
- Prevalence of rheumatic symptoms, rheumatoid arthritis, ankylosing spondylitis, and gout in Shanghai, China: A COPCORD study. J Rheumatol. 2003;30:2245-51.
- [Google Scholar]
- Epidemiological survey on prevalence of ankylosing spondylitis in 5992 Shenzhen inhabitants. Chin J Clin Rheumatol. 2006;10:159-61.
- [Google Scholar]
- Spondyloarthropathies in Japan: Nationwide questionnaire survey performed by the Japan Ankylosing Spondylitis Society. J Rheumatol. 2001;28:554-9.
- [Google Scholar]
- Age at disease onset and diagnosis delay in HLA-B27 negative vs. Positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23:61-6.
- [CrossRef] [Google Scholar]
- The potential role of genetics, environmental factors, and gut dysbiosis in the aberrant non-coding RNA expression to mediate inflammation and osteoclastogenic/osteogenic differentiation in ankylosing spondylitis. Front Cell Dev Biol. 2021;9:748063.
- [CrossRef] [Google Scholar]
- Guilt by association: HLA-B27 and ankylosing spondylitis. Immunol Today. 1990;11:137-42.
- [CrossRef] [Google Scholar]
- Endoplasmic reticulum aminopeptidases in the pathogenesis of ankylosing spondylitis. Rheumatology (Oxford). 2015;54:1549-56.
- [CrossRef] [Google Scholar]
- Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J Immunol. 2011;186:2672-80.
- [CrossRef] [Google Scholar]
- An update on the genetic polymorphism of HLA-B*27 with 213 Alleles encompassing 160 subtypes (and still counting) Curr Rheumatol Rep. 2017;19:9.
- [CrossRef] [Google Scholar]
- Defining genetic architecture of the populations in the Indian subcontinent: Impact of human leukocyte antigen diversity studies. Indian J Hum Genet. 2010;16:105-7.
- [CrossRef] [Google Scholar]
- HLA antigen and haplotype frequencies in Bhargavas and Chaturvedies of UP (India) Ind J Hum Genet. 1995;5:25-30.
- [Google Scholar]
- People of India: An investigation of biological variability in ecological ethno-economic and linguistic groups Delhi: Kamla-Raj Enterprises; 1994. p. :1-21.
- [Google Scholar]
- The prevalence of HLA-B27 in the US: Data from the US National Health and Nutrition Examination Survey, 2009. Arthritis Rheum. 2012;64:1407-11.
- [CrossRef] [Google Scholar]
- High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med. 1973;288:704-6.
- [CrossRef] [Google Scholar]
- Low frequency of HLA-B27 in ankylosing spondylitis and its relationship with clinical findings in patients from Turkey. Eur J Rheumatol. 2017;4:268-71.
- [CrossRef] [Google Scholar]
- The genetic background of ankylosing spondylitis. Joint Bone Spine. 2009;76:623-8.
- [CrossRef] [Google Scholar]
- The interplay between the geographic distribution of HLA-B27 alleles and their role in infectious and autoimmune diseases: A unifying hypothesis. Autoimmun Rev. 2009;8:420-5.
- [CrossRef] [Google Scholar]
- Diversity among selected caste and tribe groups of western Indian: Origin, HLA and disease association. Int J Hum Genet. 2004;4:105-10.
- [CrossRef] [Google Scholar]
- The genetics of spondyloarthropathies. Joint Bone Spine. 2006;73:355-62.
- [CrossRef] [Google Scholar]
- Low frequency of HLA-B27 in ankylosing spondylitis patients from Turkey. Joint Bone Spine. 2008;75:299-302.
- [CrossRef] [Google Scholar]
- Association of an extended haplotype of HLA class I alleles and their flanking microsatellites with spondyloarthropathies in South Indian patients. Hum Immunol. 2006;67:318-23.
- [CrossRef] [Google Scholar]
- Clinical correlates of HLA-B*27 and its subtypes in enthesitis-related arthritis variant of juvenile idiopathic arthritis in south Indian Tamil patients. Int J Rheum Dis. 2019;22:1289-96.
- [CrossRef] [Google Scholar]
- Correlation of HLA B27 subtypes with clinical features of ankylosing spondylitis. Int J Rheum Dis. 2011;14:369-74.
- [CrossRef] [Google Scholar]
- HLA-B27 subtypes in enthesitis-related arthritis category of juvenile idiopathic arthritis and ankylosing spondylitis in Northern India. Clin Exp Rheumatol. 2015;33:931-5.
- [Google Scholar]






