Translate this page into:
Rare presentation of gastric glomus tumor in a 28-year-old female with gastritis
*Corresponding author: Aalaa Mubarak, Department of Pathology, Salmaniya Medical Complex, Manama, Bahrain. aalaa.mubarak@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mubarak A, Alshaikh SA, AlKhuzaie J, Ali N. Rare presentation of gastric glomus tumor in a 28-year-old female with gastritis. Indian J Med Sci. 2024;76:91-4. doi: 10.25259/IJMS_193_2023
Abstract
This case report describes a rare presentation of a gastric glomus tumor in a 28-year-old Bahraini female patient who was previously diagnosed with gastritis and was on proton-pump inhibitor therapy for the past 3 years. The patient presented with symptoms of anemia and coffee ground vomiting, which led to further investigation and the eventual diagnosis of a glomus tumor in the stomach. Glomus tumors are uncommon neoplasms derived from the glomus body, a specialized arteriovenous structure involved in thermoregulation. Although they typically occur in the extremities, glomus tumors can rarely be seen in visceral organs, including the stomach. This case emphasizes the importance of considering unusual etiologies in patients with atypical presentations, even in those with pre-existing gastrointestinal conditions.
Keywords
Gastric glomus tumor
Gastritis
Coffee ground vomiting
Anemia
Proton-pump inhibitors
INTRODUCTION
Gastric glomus tumors are exceedingly rare neoplasms[1] that arise from the glomus body within the stomach. Glomus tumors are typically found in the subungual region of the extremities[2] and are rarely encountered in visceral organs. The clinical presentation of gastric glomus tumors can be non-specific, often mimicking other common gastrointestinal disorders,[1] and the radiological findings may mimic other gastrointestinal tumors, most commonly gastrointestinal stromal tumors (GIST).[2]
Here, we present a case of a 28-year-old Bahraini female patient, with a known history of gastritis and presented with low hemoglobin (Hb) level, and coffee ground vomiting, ultimately diagnosed with a gastric glomus tumor.
CASE REPORT
A 28-year-old female patient with a previous diagnosis of gastritis is on regular protein-pump inhibitor medications for 3 years duration. The patient presented to the local health center with dizziness, palpitations, abdominal pain, and heartburn for 3 days duration. The patient also stated having coffee ground vomiting for 1-day duration. On further history taking, the patient said that she has been having dark color stool for several months now, but denied any history of chest pain, significant weight loss, or fever.
Initial investigation at local health center reveal sinus tachycardia and low Hb levels of 5.2 g/dL. Hence, the patient was referred to a tertiary hospital for further investigation. On physical examination, the patient appeared pale, and her vital signs were stable. Her abdomen was soft and lax without any areas of tenderness.
The initial differential diagnoses with these presenting symptoms include gastric peptic ulcer disease, with possible perforation, aggravating of gastritis severity or a vascular neoplasm. On further medical work-up conducted, an upper gastrointestinal endoscopy was done for the patient and a submucosal bulging area was found, at the pre-pyloric region, with few clean based ulcers, one of which was oozing blood. Hence, a computed tomography (CT) scan was done, which reveal a well-defined submucosal soft-tissue mass [Figure 1] at the stomach which is protruding intraluminally and measuring 3.2 × 4 × 5 cm. The radiological differential diagnosis included GIST.

- Computed tomography scan view shows a smooth submucosal rounded heterogeneously enhancing lesion with areas of hypodensities within protruding intraluminally (blue arrows). Small calcified focus noted within.
The patient was eventually referred for the surgical department, where an exploratory laparotomy and distal gastrectomy, including removal of the gastric mass were performed.
On examining the specimen macroscopically in the histopathology department, a large oval-shaped, focally ulcerated, and polypoidal mass [Figure 2] was seen at the submucosa of the pre-pyloric area measuring around 4 × 4 × 3.2 cm. This mass was well defined, without any areas of infiltration, and it was away from all resection margins. Serial sectioning of the mass was tan, hemorrhagic, and friable in consistency.
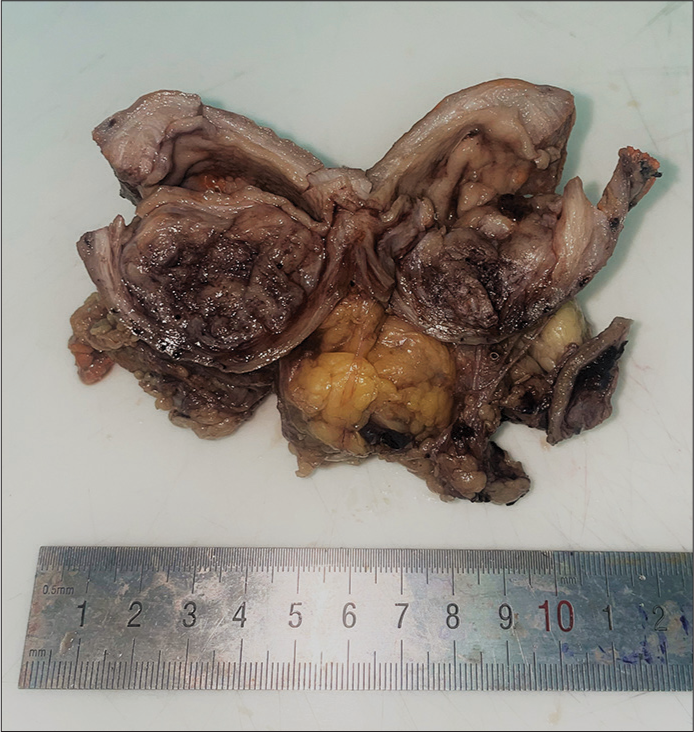
- Gross examination shows a submucosal tumor with tan hemorrhagic cut surface.
Histopathological examination of the gastric mass demonstrated an ill-defined, submucosal tumor composed of uniform, and round to polygonal cells [Figure 3] with scant cytoplasm focally showing cytoplasmic clearing [Figure 3]. The tumor also exhibit many dilated and congested vascular spaces interspersed between the tumor cells seen. There were no pleomorphic cells, increased mitotic activities, or necrosis seen.
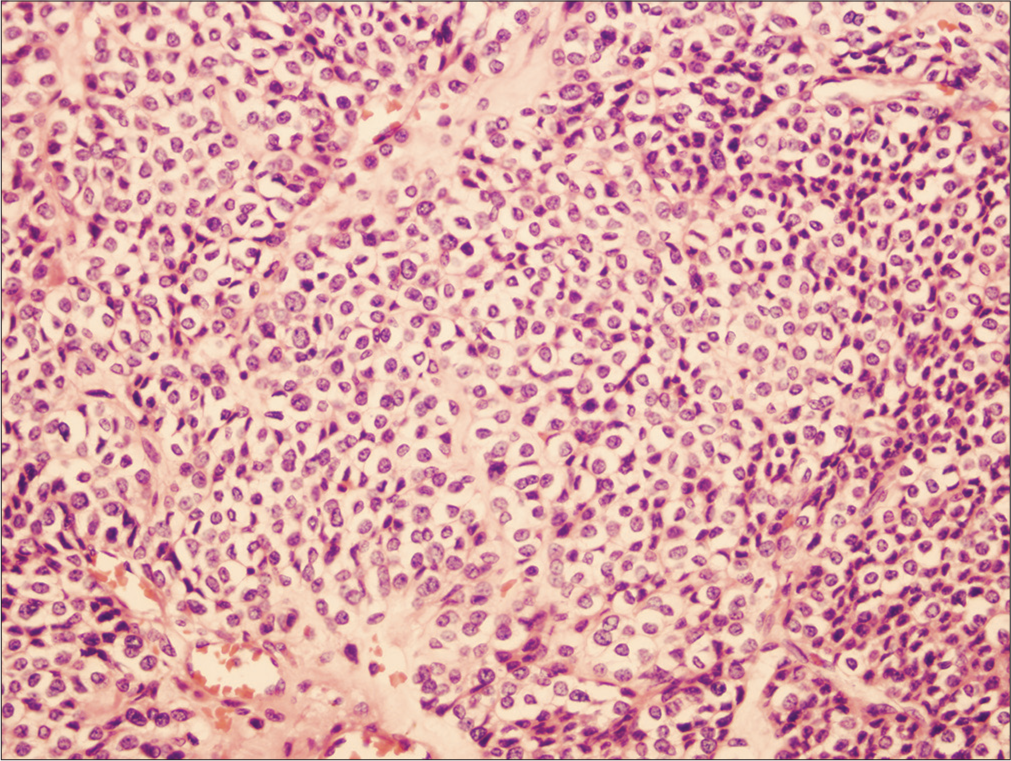
- The tumor shows large nests and sheets of uniform polygonal to round cells, containing scant cytoplasm with focal cytoplasmic clearing. The left lower periphery shows dilated blood vessels. Hematoxylin and eosin stain (H&E), magnification power 20X.
Immunohistochemical staining of the tumor cells revealed positive reactivity for smooth muscle actin (SMA) and Vimentin, [Figure 4] confirming the diagnosis of a gastric glomus tumor. Other immunohistochemical markers were all negative, they include DOG1, CD34, C-kit, synaptophysin, chromogranin, and S-100.
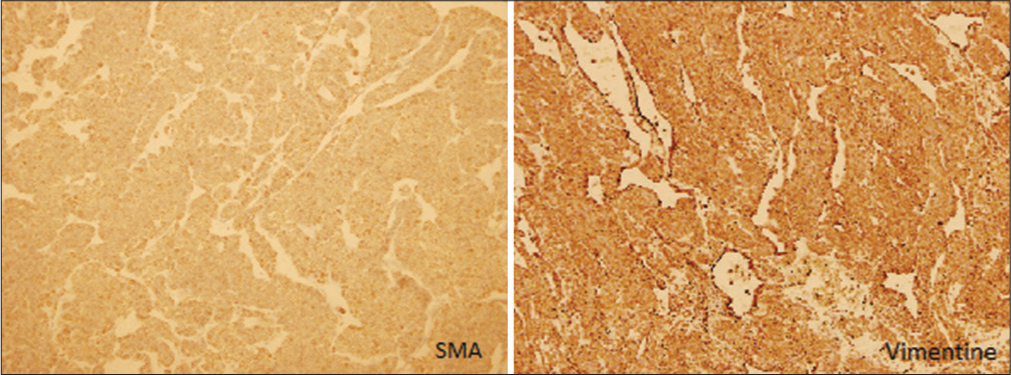
- The tumor cells are polygonal to round in shape, with scant cytoplasm and focal cytoplasmic clearing. Left side: Smooth muscle actin immunohistochemistry show diffuse positivity. Right side: Vimentin immunohistochemistry is diffusely positive. (Magnification power 5x).
The post-operative course was uneventful, and the patient’s symptoms resolved.
DISCUSSION
Glomus tumors are rare tumors[1] and represent around 2% of all soft-tissue tumor.[3] Usually, glomus tumor occurs in distal extremities and digits, as this tumor arise from glomus cells which are modified smooth muscle cells.[4] However, this tumor can infrequently grow in visceral organs, with exceedingly rare occurrence in stomach, representing approximately 1% of all mesenchymal tumors of the gastrointestinal tract.[1,5]
Not many cases of gastric glomus tumor have been reported in the literature since the first case described in 1951 by Kay et al.[5]
These tumors usually occurred in the fifth decade of life[6] with a median age of 55 years old.[5] They have significant female predominance.[5] However, few studies display equal gender distribution.[2]
The most common symptom is epigastric discomfort. Other symptoms include upper gastrointestinal bleeding,[7] which might be life threatening and can lead to anemia and melena. Other symptoms include epigastric pain,[8] nausea and vomiting. In this case, the patient initially presented with anemia and coffee ground vomiting, which is a worrisome sign of upper gastrointestinal bleeding.
A close differential diagnosis of gastric glomus tumor includes GIST. To differentiate these two entities based only on clinical and radiological features is challenging, as both tumors can appear as well circumscribed submucosal masses on CT scan, upper gastrointestinal endoscopy, and on Barium meal study,[1,2] and both might cause bleeding as a clinical symptom. Endoscopic ultrasound manifestation may detect the layer from which the tumor is arising and give a clue to the correct diagnosis. However, Tsagkataki et al. stated that there are no pathognomonic endoscopic findings that point toward glomus tumor.[6] Adequate biopsy taken through endoscopy is problematic, as the tumor is located deep and there is a high risk of bleeding.[9] Hence, the gold standard diagnostic tool is histopathological examination after surgical excision.
Glomus tumors are characterized by their distinctive histopathological features. On macroscopic gross examination, the tumors are usually well-circumscribed and have overlying ulcerations, In this case, the tumor presented as an ulcerated bulging mass. The tumor size usually ranges from approximately 2 to 3 cm[10] and on cutting open the tumor grossly, the cut surface is often tan hemorrhagic. One study conducted in 2002 by Miettinen et al. suggested that a size of more than 5 cm may indicate malignant behavior.[4,10]
On microscopic examination, glomus tumors are composed of multiple large nests and sheets of uniform, round cells, with well-demarcated cell boarder, abundant cytoplasm, and regular bland nuclei. Glomus tumors may exhibit myxoid changes centrally, and dilated vascular channels are often seen at the periphery. Occasionally, the tumor nests are separated by gastric muscularis propria. Benign gastric glomus tumors should not exhibit any nuclear atypia, necrotic areas, or any atypical mitosis.
Immunohistochemical staining for SMA and Vimentin is typically positive, supporting the diagnosis. Kamboj et al. stated that synaptophysin could shows positivity in visceral glomus tumors.[11] Nevertheless, glomus tumors of the extremities do not show such positivity for synaptophysin. Chromogranin is negative in glomus tumors.[11] This should warrant the pathologist not to misdiagnose the case as carcinoid tumor. Both of these neuroendocrine markers were negative in the above-presented case.
The prognosis for gastric glomus tumors is generally favorable, with a low incidence of recurrence or metastasis reported in the literature.[4,12] Malignant transformation is very rare in gastric glomus tumors. A specific criterion has been proposed by Folpe et al. to define malignant gastric glomus tumors and determine the risk of metastasis.[13] This criterion includes a size of more than 20 mm, a deeper seated anatomic location of the tumor, a mitotic count of more than 5/50 high power field (HPF) (>5/50 HPF), the presence of atypical mitosis, as well as moderate to severe nuclear grade.[13] On the contrary, Miettinen et al. described that few deeper seated gastric glomus tumors demonstrate a more favorable prognosis than other deep seated tumors on the peripheral extremities; therefore, the criteria of a deeper location might not be beneficial in the case of gastric glomus tumors.[4] Besides, the tumors which are 5 cm or more in size are the ones that exhibit worse prognosis; hence, it would be more applicable to use the cut point size of 5 cm rather than 2 cm as a component of malignant transformation criteria. Although all of these findings which indicate malignant behaviors are rare and most gastric glomus tumors are benign, long-term follow-up is advised to detect any of these atypical findings at an earlier stage.[14]
In the rare cases where metastasis of gastric glomus tumors has occurred, the most common sites include liver, brain, and bones. Other sites include small intestines and lungs.
The treatment of choice for gastric glomus tumor is wide local excision with safe margins. There is no indication for regional lymph node removal.[15] An alternative, less invasive procedure for excision of gastric glomus tumors, through endoscopy has also been suggested.[9] However, due to high vascularity of the tumor and increased possibility of bleeding, this suggested way of resection is not feasible.
CONCLUSION
This case highlights the importance of considering rare pathologies in uncommon anatomical sites in patients with atypical presentations, even in those with pre-existing gastrointestinal conditions such as gastritis. Gastric glomus tumors are exceedingly rare and can mimic other more common gastrointestinal mesenchymal tumors. Prompt recognition, appropriate diagnostic evaluation, and surgical intervention are crucial for achieving favorable outcomes in patients with gastric glomus tumors.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Gastric glomus tumor: A case report. World J Surg Oncol. 2010;8:19.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathologic features of gastric glomus tumor: A report of 15 cases and literature review. Pathol Oncol Res. 2022;28:1610824.
- [CrossRef] [PubMed] [Google Scholar]
- Gastric glomus tumor: A clinicopathologic and immunohistochemical study of 21 cases. Biomed Res Int. 2020;2020:5637893.
- [CrossRef] [PubMed] [Google Scholar]
- Gastrointestinal glomus tumors: A clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am J Surg Pathol. 2002;26:301-11.
- [CrossRef] [PubMed] [Google Scholar]
- Gastric glomus tumor: A case report and review of the literature. J Med Case Rep. 2021;15:415.
- [CrossRef] [PubMed] [Google Scholar]
- Glomus tumor of the stomach: A clinicopathologic analysis of 10 cases and review of the literature. Gut Liver. 2012;6:52-7.
- [CrossRef] [PubMed] [Google Scholar]
- Glomus tumor of the stomach: MRI findings. Am J Roentgenol. 2005;185:1190-2.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic diagnosis and treatment of gastric glomus tumors. Gastrointest Endosc. 2011;73:371-5.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant gastric glomus tumor: A case report and literature review of a rare entity. Oman Med J. 2016;31:60-4.
- [CrossRef] [PubMed] [Google Scholar]
- Synaptophysin positive glomus tumor of trachea simulating typical carcinoid: A potential trap. Head Neck Pathol. 2021;15:994-8.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant glomus tumor of the stomach with multiorgan metastases: Report of a case. Surg Today. 2010;40:662-7.
- [CrossRef] [PubMed] [Google Scholar]
- Atypical and malignant glomus tumors: Analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12.
- [CrossRef] [PubMed] [Google Scholar]
- Gastrointestinal glomus tumors: A single institution, 20-year retrospective study. J Surg Res. 2023;283:982-91.
- [CrossRef] [PubMed] [Google Scholar]







