Translate this page into:
The interpretation of platelet indices (platelet count, mean platelet volume, and platelet distribution width) as additional diagnostic tool for neonatal sepsis
*Corresponding author: Manisha Verma, Department of Pediatrics, Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, Uttar Pradesh, India. manisha.verma.lko@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gautam VK, Verma M, Singh A, Agrawal A, Verma A. The interpretation of platelet indices (platelet count, mean platelet volume, and platelet distribution width) as additional diagnostic tool for neonatal sepsis. Indian J Med Sci 2023;75:128-32.
Abstract
Objective:
This study aims to determine whether platelet indices (PI) (platelet count, mean platelet volume [MPV], and platelet distribution width [PDW]) are an additional diagnostic tool for neonatal sepsis (NS).
Materials and Methods:
This observational and cross-sectional study was done between April 2020 and April 2021 at the neonatal intensive care unit (NICU) of Lala Lajpat Rai Memorial Medical College Meerut, Uttar Pradesh, India. Neonates with sepsis-like apnea, abdominal distension, refusal of feed, increased pre-feed aspirates, tachycardia, hypothermia, chest retractions, lethargy, and grunting; neonates with sepsis screen positive and/or culture positive; and neonates born to mothers with sepsis risk factors were included in the study. After proper aseptic condition, the venous blood sample was collected in ethylenediaminetetraacetic acid (EDTA) vial and sent to the pathology laboratory for further analysis of complete blood count (CBC) absolute neutrophil count (ANC), total leukocyte count (TLC), C-reactive protein, platelet frequency, MPV, and PDW. The data were analyzed using SPSS v26.0.
Results:
A total of 60 babies were enrolled, of which 30 neonates with sepsis were categorized as group “cases” (n = 30), and those (n = 30) neonates without sepsis were classified as group “controls.” Platelet count (low), MPV, and PDW (high) were found to be significant predictors of NS (P < 0.05).
Conclusion:
This study reports that the frequently employed PI (MPV, platelet count, and PDW) are significant predictors of the incidence of NS in NICU settings. There was no significant change in PI in prematurity and low birth weight neonates. These platelet indicators may be used as markers to detect the incidence of NS in low-resource settings.
Keywords
Platelet indices
Platelet count
Mean platelet volume
Platelet distribution width
Diagnosis
Neonatal sepsis
INTRODUCTION
Neonatal sepsis (NS) is a significant global cause of neonatal morbidity and mortality. Recently, the global burden of disease study 2016–17 estimated that 1.3 (95% CI 0.8–2.3) million annual incident cases of NS worldwide, resulting in 203,000 (95% CI 178, 700–267,100) sepsis-attributable deaths .[1] In India, the case fatality rate for sepsis in neonates varies from 25% to 65%.[2]
It is a diagnostic difficulty; however, because overlapping signs and symptoms exclude a definite diagnosis of sepsis. As a result, we must rely on investigations to lead us. Blood culture is the most accurate approach for diagnosing whether a kid has NS. Only 20% of symptomatic newborns with suspected early-onset sepsis and 30% of neonates clinically suspected of having late-onset sepsis exhibit positive blood cultures in the neonatal intensive care unit (NICU).[3,4] On the other hand, the blood culture report is too late and cannot be depended on to make rapid opinions.
We often rely on sepsis screening to get beyond these constraints. However, its sensitivity and specificity might vary. To confidently rule out sepsis, these parameters’ negative predictive values are very low.[5,6] Sepsis is difficult to diagnose, necessitating the development of improved diagnostic measures due to the limitations of blood culture, its low positive rates, and the limited diagnostic ability of the sepsis screen in neonates.
According to studies,[7-12] individuals with NS exhibit considerable alterations in their platelet indices (PI). These studies examined mean platelet volume mean platelet volume (MPV), platelet distribution width (PDW), and platelet count. These investigations have demonstrated that in neonates with sepsis, the platelet count declines while the MPV and PDW rise.[8]
There is little research on their use in NS, even though they are a promising and practical marker. When comparing PI in infants, most research has only looked at sepsis with a positive culture; sepsis with a negative culture has yet to be considered. In predicting newborn septicemia, no data are currently available that compare PI with the sepsis screen already in place.
This study aims to fill the existing literature gap and explore whether it may be used as a tool to improve the early detection of NS.
MATERIALS AND METHODS
This observational and cross-sectional study was done between April 2020 and April 2021 at the NICU of Lala Lajpat Rai Memorial Medical College (LLRMC) Meerut, Uttar Pradesh, India, after informed consent was obtained. Ethical clearance was obtained from the Institutional Ethics Committee, LLRMC (No./SC-1/2021/202 dated January 11, 2022).
The study included neonates with signs and symptoms of sepsis-like apnea, abdominal distension, refusal of feed, increased pre-feed aspirates, tachycardia, hypothermia, chest retractions and lethargy, and grunting; neonates with sepsis screen positive and/or culture positive; and neonates born to mother with risk factors for sepsis. The study excluded neonates with congenital and acquired thrombocytopenia, such as placental insufficiency, neonatal alloimmune thrombocytopenia, TAR (thrombocytopenia with absent radius) syndrome, Trisomy 13; Trisomy 18; Trisomy 21, Turner syndrome, Noonan syndrome, Inborn error of metabolism, thrombosis, fanconi anemia, and congenital thrombocytopenia. Controls were selected from healthy neonates delivered during the study period those not having features of sepsis.
After proper aseptic condition, the venous blood sample was collected in EDTA VIAL and sent to the pathology laboratory for further analysis of complete blood count (absolute neutrophil count [ANC], total leucocyte count [TLC]), CBC (ANC, TLC), C-reactive protein (CRP), Platelet frequency, MPV, and PDW.
Statistical analysis
Data were entered in M.S. Excel. Statistical analysis was conducted with S.P.S.S. v26.0. Frequency and proportions were used to express the categorical variables. The Kolmogorov–Smirnov test did not usually distribute the continuous variables (P < 0.05). Median and interquartile range (IQR) were calculated for the continuous variables. Mann–Whitney test was used to determine the significance of the difference between the cases (sepsis neonates) and controls (normal neonates).
RESULTS
A total of 60 babies were enrolled, of which 30 neonates with sepsis were categorized as group “cases” (30), and those (30) neonates without sepsis were categorized as group “controls.” Most of the neonates were males (n = 41; 68.3%) and newborns (n = 40; 66.7%). The prevalence of low birth weight was 36.7% and pre-term was 31.7%.
Clinical characteristics were seen in 50% of the neonates who had sepsis. Respiratory distress was the most prevalent clinical symptom (45%), followed by lethargy, reluctance to feed (11.7% each), and hypothermia (6.7%).
Blood cultures were positive in 16.7% (n = 10) of the neonates, with Escherichia coli being the most frequent organism (60%), followed by Staphylococcus aureus (20%) and Klebsiella (20%).
The cases and controls had median platelet counts of 80000/µL (IQR 60000–165000/µL) and 280000/µL (207500– 343750/µL), respectively. Platelet count was considerably lower in sepsis cases than in controls (P < 0.05). The median MPV was found to be 13 fL (IQR 9.8–14 fL) and 9 fL (IQR 8–11 fL) for the cases and controls, respectively. MPV was significantly higher in the sepsis cases than in the control (P < 0.05). The median PDW was found to be 19 fl (IQR 13.8–23.3 fl) and 13 fl (IQR 12–14 fl) for the cases and controls, respectively. PDW was significantly higher in the sepsis cases than in the control (P < 0.05). The median CRP was found to be 24 mg/L (IQR 9.5–54 mg/L) and 8 mg/L (IQR 6–9 mg/L) for the cases and controls, respectively. CRP was significantly higher in the sepsis cases than in the control (P < 0.05). Birth weight, TLC, Gestational age, TLC, and ANC were comparable between the cases and controls (P > 0.05). Platelet count (low), MPV, and PDW (high) were found to be significant predictors of NS (P < 0.05) [Table 1].
| Variables | Cases | Controls | P-value | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Birth weight (kg) | 2.6 | 2,3.1 | 2.8 | 2.2, 3 | 0.067 |
| Gestational age (weeks) | 38 | 36, 39 | 38 | 36, 39 | 0.089 |
| TLC | 11000 | 7750, 18000 | 9100 | 8300, 10100 | 0.093 |
| ANC | 5900 | 3150, 9650 | 4380 | 3800, 5200 | 0.054 |
| CRP | 24 | 9.5, 54 | 8 | 6, 9 | <0.001 |
| Platelet frequency | 80000 | 60000, 165000 | 280000 | 207500, 343750 | <0.001 |
| MPV (fL) | 13 | 9.8, 14 | 9 | 8, 11 | 0.003 |
| PDW (fL) | 19 | 13.8, 23.3 | 13 | 12, 14 | <0.001 |
IQR: Interquartile range, TLC: Total leucocyte count, ANC: Absolute neutrophil count, CRP: C-reactive protein, MPV: Mean platelet volume, PDW: Platelet distribution width, P<0.05 significant value
Based on Youden index, optimum cutoff of platelet count to predict the sepsis is 170,000/µL, with sensitivity of 76.7% and specificity of 96.7% [Figure 1].
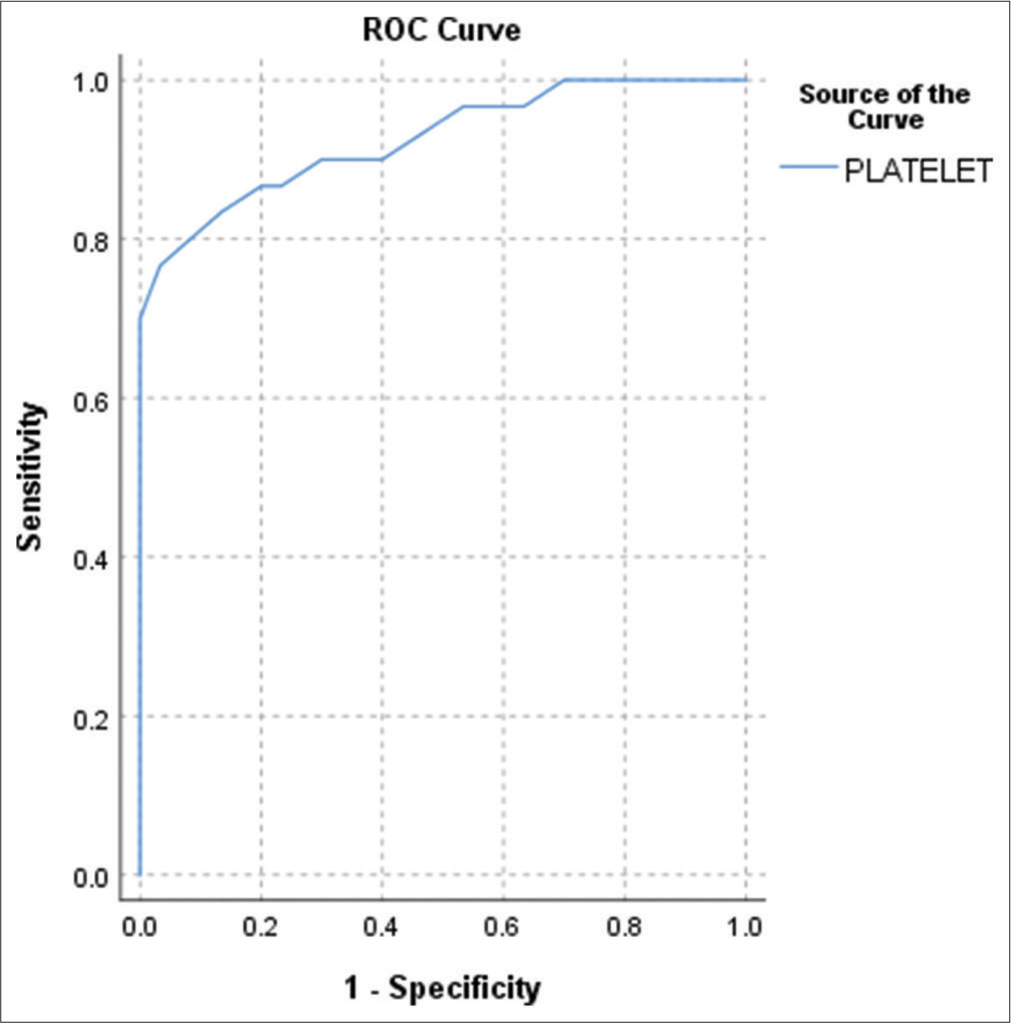
- Receiver operating characteristic curve for predicting the diagnostic accuracy of platelet count for sepsis. Area under the curve – 0.926 (P < 0.001).
Based on the Youden index, the optimum cutoff of MPV to predict sepsis is 11.5, with a sensitivity of 70% and specificity of 80% [Figure 2]. Platelet count, MPV, and PDW were insignificant predictors of low birth weight and prematurity (P > 0.05). There was a significant negative correlation between the platelet count and the CRP of the studied neonates (P < 0.05) [Table 2].
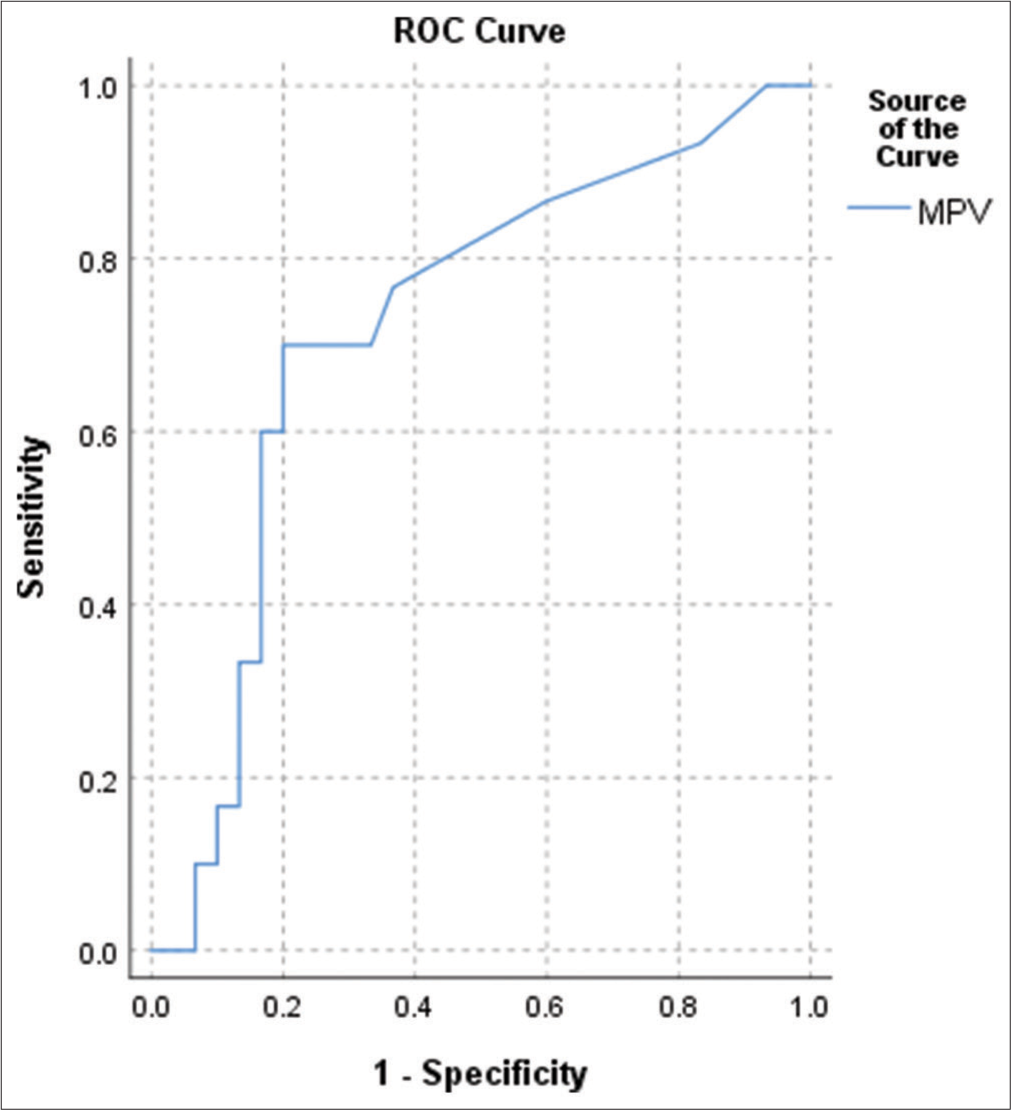
- Receiver operating characteristic curve for predicting the diagnostic accuracy of mean platelet volume for sepsis. Area under the curve – 0.722 (P = 0.001).
| Spearman correlation coefficient | P-value | |
|---|---|---|
| TLC | -0.177 | 0.176 |
| ANC | -0.228 | 0.080 |
| CRP | -0.596 | <0.001 |
TLC: Total leucocyte count, ANC: Absolute neutrophil count, CRP: C-reactive protein, P<0.05 significant value
There was a significant positive correlation between the MPV and the TLC and CRP of the studied neonates (P < 0.05) [Table 3].
| Spearman correlation coefficient | P -value | |
|---|---|---|
| TLC | 0.460 | <0.001 |
| ANC | 0.098 | 0.456 |
| CRP | 0.643 | <0.001 |
TLC: Total leucocyte count, ANC: Absolute neutrophil count, MPV: Mean platelet volume, CRP: C-reactive protein, P<0.05 significant value
There was a significant positive correlation between the PDW and the TLC and CRP of the studied neonates (P < 0.05) [Table 4].
| Spearman correlation coefficient | P -value | |
|---|---|---|
| TLC | 0.288 | 0.026 |
| ANC | 0.216 | 0.097 |
| CRP | 0.723 | <0.001 |
TLC: Total leukocyte count, ANC: Absolute neutrophil count, CRP: C-reactive protein, PDW: Platelet distribution width, P<0.05 significant value
DISCUSSION
Sepsis in neonates is a common complication and is often mortal. The present study looked at the relevance of PI, namely, platelet count, MPV, and PDW, in predicting the occurrence of NS in a tertiary care referral hospital setting in North India.
In the present study, the median (IQR) platelet count was reported to be low and high for the cases and controls. This was similar to the finding of Guida et al.,[13] who reported 54% prevalence among culture-proven sepsis, similar to our results. In contrast, Wasiluk et al.[14] and Aydemir et al.[15] reported a median platelet count of 249.0 × 103 cu mm and 225.0 × 103 cu mm, which was higher than the count reported in the present study. Yet, the platelet counts were significantly lower than the controls (normal neonates) in Aydemir et al.,[15] similar to the present study findings.
Platelet count (low), MPV, and PDW (high) were significant predictors of NS in the present study, affixing the findings of the existing literature. In this study, the optimum cutoff of platelet counts to predict sepsis is 170,000/µL, with a sensitivity of 76.7% and specificity of 96.7%.
The median MPV was found to be 13 fL (IQR 9.8–14 fL) and 9 fL (IQR 8–11 fL) for the cases and controls, respectively. MPV was significantly higher in the sepsis cases than in the control (P < 0.05). Based on Youden index, optimum cutoff of MPV to predict the sepsis is 11.5, with sensitivity of 70% and specificity of 80%. Although Aydemir et al. also reported lower MPV values (8.4) than our cases, they found that cases had significantly higher MPV in comparison with the control, which supported our findings.[15]
The median PDW was found to be 19 fL (IQR 13.8– 23.3 fL) and 13 fL (IQR 12–14 fL) for the cases and controls, respectively. PDW was significantly higher in the sepsis cases than in the control (P < 0.05). Based on Youden index, optimum cutoff of PDW to predict the sepsis is 16.5, with sensitivity of 70% and specificity of 100%. Ahmad and Waheed reported a median PDW value of 12.3 among the sepsis neonates, much lower than the present study.[16]
A sepsis screen with PDW + MPV or one with platelet count + PDW + MPV has high specificity. However, a few papers have only studied the relationship between the sepsis screen and the PI for NS evaluation.[17] However, the findings of the present investigation indicate that a combination of PI plus a sepsis screen may adequately rule out sepsis.
Limitations of the study
It was a cross-sectional study; hence, the temporal association between the PI and the thrombocytopenia could not be established. The external validity of the results is limited since the study included participants from a single center. Potential confounders like procalcitonin and time of onset of sepsis were not evaluated.
CONCLUSION
Thus, this study reports that the employed PI (MPV, platelet count, and PDW) are significant predictors of the incidence of NS in NICU settings. These platelet indicators may be used as markers to detect the incidence of NS in low-resource settings. Blood culture positivity was low among the studied sepsis, thus indicating a need to conduct studies after classifying the neonates as probable and culture-proven sepsis. Therefore, further multi-centric and prospective studies need to be undertaken between culture-proven and probable sepsis neonates to understand better and apply this study’s results.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries 1990-2013: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2015;386:743-800.
- [Google Scholar]
- A bedside prediction-scoring model for late-onset neonatal sepsis. J Perinatol. 2005;25:778-83.
- [CrossRef] [PubMed] [Google Scholar]
- Predictive clinical scores for diagnosis of late onset neonatal septicemia. J Trop Pediatr. 2003;49:235-9.
- [CrossRef] [PubMed] [Google Scholar]
- Sepsis screen in neonates with evaluation of plasma fibronectin. Pediatr Infect Dis J. 1987;6:443-6.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of bacteraemia on an automated platelet measurement in neonates. Am J Clin Pathol. 1990;93:391-4.
- [CrossRef] [PubMed] [Google Scholar]
- Frequency and mechanism of neonatal thrombocytopenia. J Pediatr. 1986;108:749-55.
- [CrossRef] [PubMed] [Google Scholar]
- Immune thrombocytopenia in severe neonatal infections. J Pediatr. 1981;98:449-53.
- [CrossRef] [PubMed] [Google Scholar]
- The mean platelet volume (MPV) in the neonatal period. Am J Perinatol. 1986;3:1-3.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for nosocomial sepsis in newborn intensive and intermediate care units. Eur J Pediatr. 1996;155:315-22.
- [CrossRef] [PubMed] [Google Scholar]
- The neonatal “sepsis work-up”: Personal reflections on the development of an evidence-based approach toward newborn infections in a managed care organization. Pediatrics. 1999;103:360-73.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet count and sepsis in very low birth weight neonates: Is there an organism-specific response? Pediatrics. 2003;111:1411-5.
- [CrossRef] [PubMed] [Google Scholar]
- The cut-off levels of procalcitonin and C-reactive protein and the kinetics of mean platelet volume in preterm neonates with sepsis. BMC Pediatr. 2018;18:253.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet counts, MPV and PDW in culture proven and probable neonatal sepsis and association of platelet counts with mortality rate. J Coll Physicians Surg Pak. 2014;24:340-4.
- [Google Scholar]
- Evaluation of platelet indices as additional diagnostic tool for neonatal sepsis. Astrocyte. 2018;4:205-9.
- [CrossRef] [Google Scholar]






