Translate this page into:
Current status of treatment of latent tuberculosis infection in India

*Corresponding author: Dr. A. Kumar, Department of Medicine, All India Institute of Medical Sciences, Teaching Block, 3094A, Ansari Nagar, New Delhi - 110 029, India. linktoarvind@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Saha S, Kumar A, Saurabh K, Shankar SH, Kashyap A, Nischal N, et al. Current status of treatment of latent tuberculosis infection in India. Indian J Med Sci 2019;71(2):54-9.
Abstract
In view of the high burden of latency of tuberculosis (TB) in India, tackling latent TB in the right way is a menace. All latent TB’s infection (LTBI) are treated in countries having low burden such as the United States. However, this approach cannot be implemented in high burden countries like India until concrete evidence or consensus by experts on this subject is made. There are very specific risk groups where these patients are to be treated as far as current evidence-based medicine is concerned. Hence, the need to develop a document was felt, through which the treatment of LTBI becomes homogeneous by each and every physician who is practicing and treating TB. The last attempt to review the topic was made in 2015, after which there have been many changes and update in this subject.
Keywords
Latent tuberculosis infection
Tuberculin skin test
Interferon-gamma release assay
INTRODUCTION
Latent tuberculosis infection (LTBI) is the persistence of an immunological response to Mycobacterium tuberculosis antigen stimulation without any clinically active disease.[1] The global prevalence of LTBI is estimated to be nearly 33%.[2] In India, there are no estimates regarding the prevalence of LTBI in the general population; however, the WHO data indicate that roughly 3.5 lakh children below the age of 5 years were eligible for LTBI treatment.[3] Although most of the infected persons do not manifest the disease, they are at high risk of developing active infection and hence represent a reservoir of bacteria. The lifetime risk of reactivation of TB is estimated to be around 5–10%.[4] This risk is much higher in those with HIV, with a 10% annual risk of reactivation, and in young children (~10%). If untreated, 40% of LTBI children under 1 year of age develop active disease, whereas it is 24% in children of 1–10 years and 16% in those between 11 and 15 years.[4-6] It has been proposed that the infected persons accumulate in the pool of LTBI from which individuals having latent TB exit with active TB. To control the active infection, the shrinking of the magnitude of the pool of latent infection is required.[7]
There has been a lot of emphasis in the past few decades on the elimination of TB. While treatment of active disease is by far the major intervention in this regard, LTB treatment forms an important yet undervalued facet. The diagnosis and treatment of LTBI are hindered by the cost implications of testing, lack of a consensus on the tests recommended, and side effects of treatment. Treatment of LTBI in low prevalence (high to upper-middle-income) countries is feasible, as elimination of this reservoir of infection will reduce the burden of the disease. However, the scenario in high prevalence countries such as India is quite the opposite. Here, reinfection due to contact with active cases rather than reactivation contributes to a high disease burden. This is the reason for the absence of a nationwide policy on LTBI treatment. In such a situation, LTBI treatment needs to be individualized. Preference should be given to those at high risk of reactivation, especially due to the short term reversible predisposing factor. Hence, the decision to treat LTBI should be taken by considering the probability of reactivation versus reinfection.
The last review of this topic was done in India by Agarwal in 2005.[8] Since then, there have been major changes in guidelines pertaining to this subject. In this review, we will focus on current evidence of LTBI testing and treatment in high incidence countries such as India.
RECOMMENDATIONS FOR LTBI TESTING
LTBI diagnosis lacks a gold standard test in today’s scenario. The mainstay of testing is through tuberculin skin test (TST) and interferon-gamma release assay (IGRA).
TST identifies people with the previous sensitization to tubercular antigens by stimulating a delayed-type hypersensitivity reaction. Time to positivity varies from 2 to 8 weeks after exposure. Interpretation of TST is based on the pre-test probability, patient profile, and setting in which the test has been performed. Cutoffs of 5, 10, and 15 mm are used. Higher cutoff increases the specificity but decreases the sensitivity of the test. Thus, the high cutoff is useful in low prevalence settings, and in those with high nontubercular mycobacterium (NTM) exposure.
Two important reasons for false-positive TST are Bacillus Calmette–Guérin (BCG) vaccination and NTM infection [Table 1]. The effect of vaccination on TST positivity varies with time of vaccination. Those vaccinated at infancy may have low-level reactivity (<10 mm) especially after 10 years or more. However, those who were vaccinated after infancy have a false positivity rate of >20% after 10 years of vaccination. The role of NTM in positivity is significant only in developed countries with low incidence and prevalence of TB.[9] TST is useful for serial testing in healthcare workers, close contacts of pulmonary TB cases in low burden countries. Those with baseline positivity (within 2.5 weeks of exposure) are considered to have prior LTBI. If negative, a second test is performed at 8 weeks after the end of the exposure. TST positivity then is considered as recent TST conversion and should be treated.
| False positive | False negative | |
|---|---|---|
| TST | Previous BCG vaccination Nontubercular mycobacterium infection | Within 6–8 weeks of infection Recent viral or bacterial illness Recent viral vaccination Immunosuppression Overwhelming infection (disseminated/extensive TB) Infants, young children |
| IGRA | Technical errors | Immunosuppression Anergy Technical errors |
TST: Tuberculin skin test, IGRA: Interferon-gamma release assay, BCG: Bacillus Calmette–Guérin, TB: Tuberculosis
As per CDC recommendations, TST positivity can be interpreted for different patient groups in ≥5 mm group, ≥10 mm group, and ≥15 mm group. Patients with HIV co- infection, recent TB contacts, post organ transplant and those taking prednisolone >15mg/day or other immunosuppressive drugs for more than a month are considered positive if TST >5mm. Whereas children immigrants (<5 years) from high TB burden countries, intravenous drug addicts, resident/employees of high risk congregate settings (prison, nursing home, health care facility, etc.), mycobacterium laboratory personnel, patients with diabetes mellitus, chronic renal failure, silicosis, leukemia, lymphoma, head and neck cancer, and gastrectomy are considered positive if TST ≥10 mm. Those patients who are not at risk of TB on clinical history and evaluation are considered to be TST positive if it is ≥15 mm.[10]
The IGRAs are in vitro tests that quantify the response of lymphocytes when exposed to M. tuberculosis antigens. They are of two types: QuantiFERON TB gold in-tube test (QFT-GIT) and T-SPOT. The QFT-GIT uses ELISA, whereas the T-SPOT uses enzyme-linked immuno-spot. A new QFT (QFT Plus) has been released in Europe but is less studied.
The antigens used in IGRAs (ESAT-6, CFP-10, and TB7.7) are present in M. tuberculosis and wild type Mycobacterium bovis, but absent in BCG strains of M. bovis and NTM species, except Mycobacterium kansasii, Mycobacterium szulgai, Mycobacterium leprae, and Mycobacterium marinum. This confers higher specificity as compared to TST, especially in BCG vaccinated persons, and in low burden countries with high background NTM infections. IGRA tests are reported as positive, negative, or indeterminate/borderline. There is modest variability in the results, which increases at either end of the spectrum. Immunosuppressed patients and HIV positive are more likely to have indeterminate results [Table 1]. Here, repeat testing with IGRA/TST may be of use.
There is no data regarding the preference of one IGRA assay over another, except in HIV, where the T-SPOT has yielded better results.
A novel form of the TB skin test, the C-TB test, is currently under trial. This test is similar to the TST but contains the purified antigens CFP10 and ESAT-6. This combines the cost-effectiveness and the ease of the TST with the specificity of the IGRAs in the diagnosis of LTBI. The C-TB test is also unaffected by BCG vaccination. This test has fared well in Phase 3, double-blinded, and randomized trial published in 2017. It showed 94% concordance with the IGRA results with similar indurations sizes as the TST.[11]
RECOMMENDATIONS REGARDING TESTING FOR LTBI ARE AS FOLLOWS:[2]
Testing for LTBI should only be done in those who are likely to have the infection
Before testing, all patients must be systematically investigated for the active tuberculous disease
According to the WHO, in high to upper-middle- income countries with a TB incidence of <100 per 100 000, TST or IGRA may be used. In low to middle- income countries such as India, TST is preferred due to comparable performance and lower cost of TST vis-à-vis IGRA[12]
The CDC recommends a three-pronged approach to testing and treating LTBI [Figure 1]. It takes into consideration the risk of infection, the likelihood of progression of the disease, and the likely benefit of therapy. It recommends the use of IGRA over TST in all situations, except in children <5 years (TST) and in high-risk patients (no specific recommendation)
TST should not be replaced by IGRA in children, especially in high burden countries. However, the two tests may be complementary to each other in improving the sensitivity and specificity of the results[13,14]
An algorithm for the testing and treatment of LTBI is shown in Figure 2. This has been adopted from 2018 WHO LTBI treatment guidelines.[15]
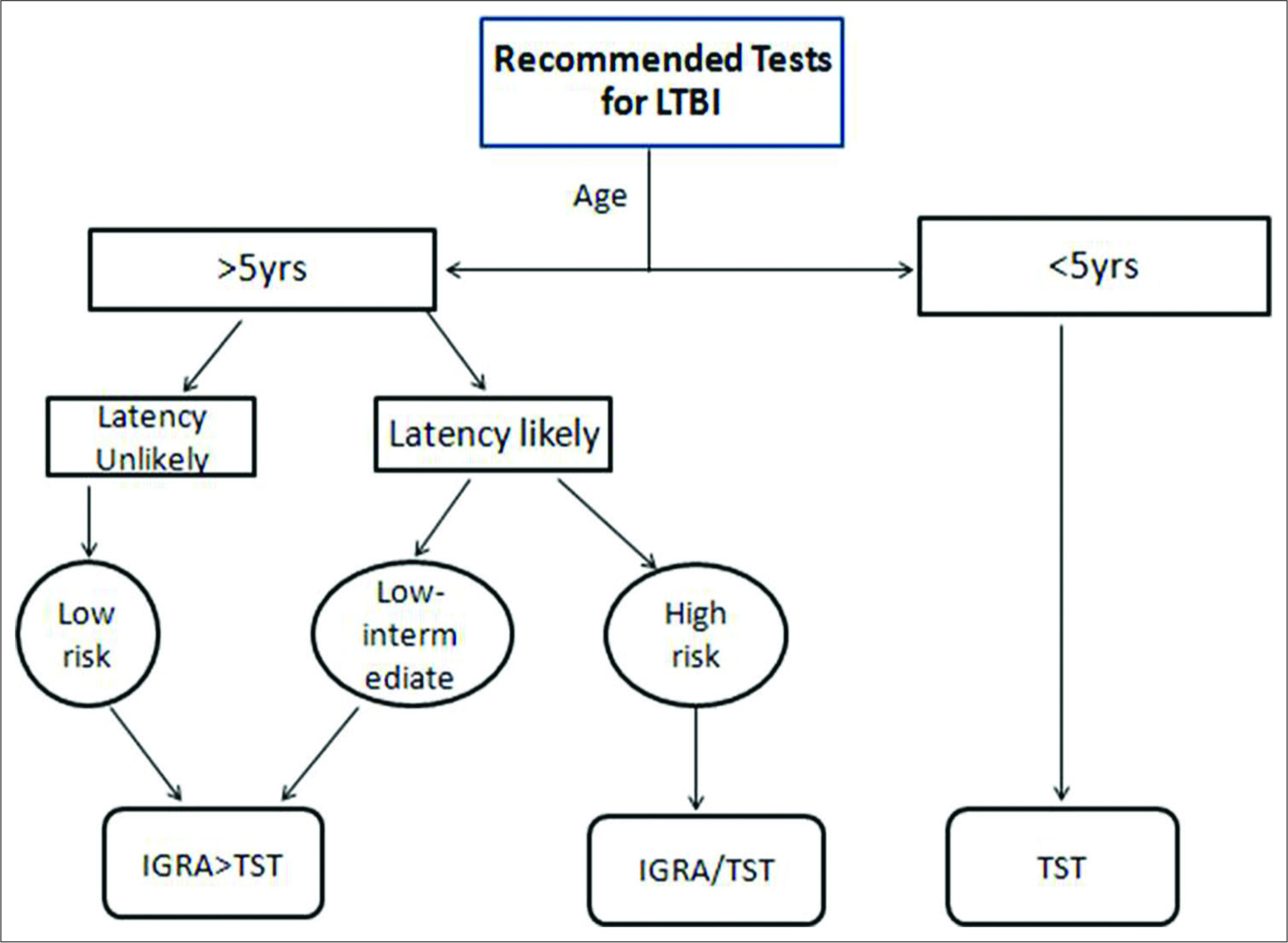
- Recommended approach to test for latent tuberculosis infection (modified from CDC and WHO guideline). In the Indian context, tuberculin skin test may be preferred over interferon-gamma release assay (refer text).
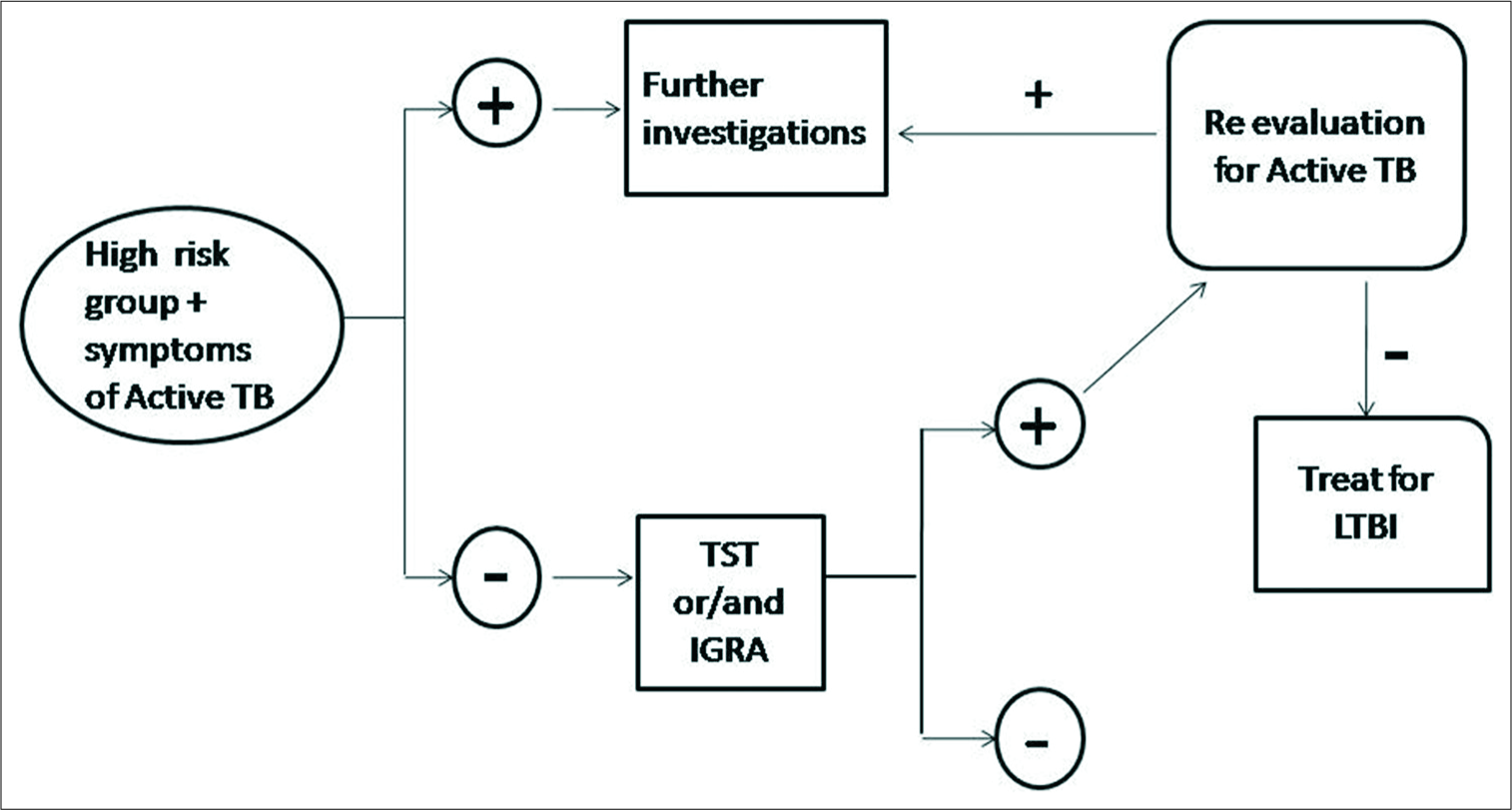
- Algorithm for the testing and treatment of latent tuberculosis infection.
The WHO recommendations for patient groups in whom LTBI testing should be done are similar to CDC recommendations for the high to upper-middle-income countries with low TB incidence (<100 per 100,000). This includes HIV, contacts of active cases, patients on dialysis, antitumor necrosis factor (TNF) therapy, and immunosuppressed, patients with silicosis those living in close conditions include prisons and nursing homes. Countries with limitation of resources, middle income, and with high TB burden (like India), testing is recommended only in HIV, and children <5 years of age.[16] A new recommendation as per the WHO LTBI guidelines (2018), states that children >5 years, adolescents, and adults who are household contacts of microbiologically proven TB may be screened for LTBI after screening for active infection.[15] Table 2 gives the guidelines for LTB testing and treatment in various countries.
| USA-CDC | UK-NICE | Canada | Australia | Japan | Phillipines | |
|---|---|---|---|---|---|---|
| Year | 2016 | 2016 | 2013 | 2015 | 2014 | 2016 |
| Testing recommended in | Likely to have a disease | Close contacts, immunosuppressed, native of high burden countries, etc. | High risk | High risk | High risk | High risk |
| Test | IGRA>TST in all TST in <5 years | TST±IGRA (separate recommendations for each cohort) | TST | TST IGRA in previously BCG vaccinated |
TST/IGRA | TST |
| Treatment | HR for 3 months if hepatotoxicity is a concern H for 6 months |
H 9 months | HR 3 months H 6 months R 4 months if H resistance/intolerance |
H 6–9 months>R 4–6 months | H 6 months(DOT) |
TST: Tuberculin skin test, IGRA: Interferon-gamma release assay
TESTING IN PATIENTS ON IMMUNOSUPPRESSION
It is a well-known fact that people on immunosuppressant drugs such as steroids, biologicals, disease-modifying antirheumatic drugs, and other antirheumatic drugs have a greater risk of developing TB than the general population. This risk is highest in those taking TNF-alpha inhibitors, with a relative risk (RR) ranging from 1.7 to 9 followed by glucocorticoids (RR 4.9).[13,20] There are several recommendations regarding LTBI management in these patients.
The ACR guidelines published in 2015 for rheumatoid arthritis (RA) treatment recommend testing and treating LTBI in patients receiving biologics. However, LTBI screening may be omitted if using B cell agents (like rituximab). It recommends IGRA over TST only in persons with prior BCG vaccination. For the rest, either test can be used. As the TST can cause IGRA positivity, IGRA must always precede TST testing. Repeat testing is recommended in immunosuppressed, and those with high-risk conditions (as given by the CDC). Treatment for LTBI must be given to all who are either IGRA/TST positive and without active TB. After at least 1 month of treatment, biological can be given. If biological therapy must be continued, then LTBI screening may be considered on an annual basis if the patient initially tested negative.[18,22-24]
In India, although a large number of patients suffer from rheumatic diseases, there are no clear guidelines for LTBI testing and treatment. The value of TST is doubted, due to false negativity in immunosuppressed patients, and false positivity due to BCG vaccination. Even in populations with low TB incidence, TST becomes positive after stopping steroids for at least 1 month and after 3 months with other immunosuppressants.[25,26] The role of IGRA is not well established in India. It may be prudent to start all patients planned for anti-TNF therapy on LTBI treatment after judicious interpretation of TST/IGRA. This is the current practice at most rheumatology centres. Those with radiographic scarring without anti-tubercular treatment (ATT) intake and those with inadequate ATT intake in the past should be offered chemoprophylaxis. Patients with adequately treated TB in the past may be kept under close monitoring with 3 monthly radiographs.[27] Indian Academy of Pediatrics (IAP) recommends that TST should be done on every child who is receiving steroid therapy, e.g., children suffering from acute leukemia and nephrotic syndrome.[22]
TESTING IN HIV
The guidelines given by the WHO for HIV patients in resource-limited settings recommend the initiation of isoniazid preventive therapy (IPT) in all individuals (children >1 year, adolescents, adults, and pregnant women) as a part of a comprehensive care package, irrespective of the degree of immunosuppression. TST is not compulsory to initiate IPT; however, those with a positive TST benefit more from it. Previously treated TB patients, those on ART and pregnant women should also receive IPT. The recommendation in children <1 year states that IPT should be given only in those who are close contacts of active TB cases. There is a conditional recommendation to give 6 months IPT in children after completion of chemotherapy for active TB. In all subsets, at least 6 months of IPT is preferred. In high burden countries, 36 months of IPT can be considered in lieu of lifelong therapy. The caveat to all these recommendations is to look for and exclude active TB before initiating IPT. At every contact with the patient, screening for TB must be done.[28]
TREATMENT OF LATENT TB
Chemotherapy of LTBI is the only biomedical TB control intervention because it can sterilize latent infection. The IAP has recommended isoniazid for treatment of LTBI in the dose of 10 mg/kg/day for 6 months. The treatment should be given to:
Asymptomatic contacts (under 6 years of age) of a smear-positive case, without evidence of active disease, should be given in regardless of their TST, BCG, or nutritional status
All HIV positive children in contact with an infectious TB case or TST positive (≥5 mm induration), after ruling out active TB
TST positive children planned for or receiving immunosuppression (e.g., acute leukemia and nephrotic syndrome)
A child born to TB positive mother if there is no evidence of congenital TB in the new-born.[29]
The recommendation in adults is to treat patients with RA and LTBI who are planned for immunosuppression (biologicals). This is in keeping with the ACR guidelines.[23] In HIV, chemotherapy is initiated as described above. However, due to lack of clear cut guidelines for India, treatment in other cases such as immunosuppressed and close contacts of active cases are done on a case to case basis.
The treatment options available for treating LTBI are isoniazid monotherapy for 6 months, rifampicin and isoniazid combination daily for 3 months (in children <15 years), rifapentine and isoniazid weekly for 3 months, in countries with high incidence of TB isoniazid is given at a dose of 5 mg/kg in adults, and 10 mg/kg in children up to a maximum of 300 mg. Rifampicin, when used, is given at a dose of 10 mg/kg in adults and 15 mg/kg in children up to a maximum of 600 mg.[15]
In most studies conducted to date, all the above regimens were non-superior to each other. However, on a case to case basis, some regimens may be preferred over others. For example, in HIV patients, rifapentine/rifampicin containing regimens are not preferred due to the high risk of drug interactions. In others, these may be preferred as they are shorter and patients are likely to be more compliant. However, cost implications must be considered as well.
CONCLUSION
TB is a major burden in our nation. The emphasis of nationwide programs is on treating active TB cases; however, the pool of LTBI is an important one. The selection of the LTBI subgroup requiring management is an under-learn aspect of comprehensive patient care. This must be offered to high-risk individuals. Currently, LTBI treatment is recommended in children below 6 years of age who are contacts of smear-positive cases, born to mothers with TB, are immunosuppressed with TST positive, or in contact with active TB cases. In adults with HIV, a personalized approach to care is taken. All HIV patients, including pregnant women, whether TST positive or negative, are candidates of IPT. However, the benefit of treatment may be more in those with TST positivity. RA patients with TST/IGRA positivity planned for biologics are treated for LTBI.
While the results of treating LTBI at a nationwide level may not be evident in the short run, it will decrease the reservoir of infection which will impact future elimination efforts. The available data on testing for LTBI as well as treatment outcomes in India are sadly lacking. Being a high burden country, such data are highly relevant. We also need to identify other high-risk subgroups where LTBI treatment may be warranted. The complex nature of the disease and various socioeconomic factors mandates further research in this area.
Declaration of patient consent
Not required as there are no patients in this study
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- LTBI: Latent tuberculosis infection or lasting immune responses to M.tuberculosis? A TBNET consensus statement. Eur Respir J. 2009;33:956-73.
- [CrossRef] [PubMed] [Google Scholar]
- Consensus statement. Global burden of tuberculosis: Estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677-86.
- [CrossRef] [PubMed] [Google Scholar]
- CSV Files, World Health Organization Latent TB Estimates. 2015. Available from: http://www.who.int/tb/country/data/download/en [Last accessed on 2017 Mar 20]
- [Google Scholar]
- The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol. 1974;99:131-8.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberculosis (TB) Australian Government Department of Health. Available from: http://www.health.gov.au/internet/main/publishing.nsf/content/cdna-songtuberculosis#_ENREF_15 [Last accessed on 2017 Jan 10]
- [Google Scholar]
- Tuberculosis: A Comprehensive International Approach (2nd ed). Boca Raton: CRC Press; 2000. p. :865.
- Latent tuberculosis in children: Diagnosis and management. Indian J Pediatr. 2011;78:464-8.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of latent tuberculous infection in India: Is it worth the salt? Lung India. 2005;22:105.
- [Google Scholar]
- False-positive tuberculin skin tests: What is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192-204.
- [Google Scholar]
- Targeted Tuberculin Testing and Treatment of Latent Tuberculosis Infection. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr4906a1.htm [Last accessed on 2016 Dec 27]
- [Google Scholar]
- Safety and efficacy of the C-Tb skin test to diagnose Mycobacterium tuberculosis infection, compared with an interferon? release assay and the tuberculin skin test: A phase 3. double-blind, randomised, controlled trial. Lancet Respir Med. 2017;5:259-68.
- [CrossRef] [Google Scholar]
- Information NC for B, Pike USNL of M 8600 R, MD B, USA 20894. Use of Tuberculosis Interferon-Gamma Release Assays (IGRAs) in Low and Middle Income Countries: Policy Statement.
- [Google Scholar]
- Treatment of latent tuberculosis infection: An update. Respirology. 2010;15:603-22.
- [CrossRef] [PubMed] [Google Scholar]
- Updated Guidelines for Using Interferon Gamma Release Assays to Detect Mycobacterium tuberculosis Infection---United States. 2010. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5905a1.htm [Last accessed on 2017 Apr 04]
- [Google Scholar]
- Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management, Licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization; 2018.
- [Google Scholar]
- Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46:1563-76.
- [CrossRef] [PubMed] [Google Scholar]
- Official American Thoracic Society/ Infectious Diseases Society of America/Centers for disease control and prevention clinical practice guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111-5.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberculosis Recommendations Guidance and Guidelines. Available from: https://www.nice.org.uk/guidance/ng33/chapter/recommendations [Last accessed on 2017 Jan 03]
- [Google Scholar]
- Canadian Respiratory Guidelines. Available from: http://www.respiratoryguidelines.ca/guideline/infectious-respiratory-diseases [Last accessed on 2017 Jan 10]
- [Google Scholar]
- Philippine College of Chest Physicians. Available from: http://www.philchest.org/publications/clinical-practice-guidelines [Last accessed on 2017 Jan 10]
- [Google Scholar]
- Antirheumatic drugs and the risk of tuberculosis. Clin Infect Dis. 2006;43:717-22.
- [CrossRef] [PubMed] [Google Scholar]
- 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:1-25.
- [CrossRef] [PubMed] [Google Scholar]
- Guidance for the management of patients with latent tuberculosis infection requiring biologic therapy in rheumatology and dermatology clinical practice. Autoimmun Rev. 2015;14:503-9.
- [CrossRef] [PubMed] [Google Scholar]
- BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax. 2005;60:800-5.
- [CrossRef] [PubMed] [Google Scholar]
- British Thoracic Society (BTS) recommendations for assessing risk and managing tuberculosis in patients due to start anti-TNF-{alpha} treatments. Rheumatology (Oxford). 2005;44:1205-6.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for tuberculosis prophylaxis during anti-tumour necrosis factor-a treatment: Indian rheumatology association. APLAR J Rheumatol. 2006;9:181-3.
- [CrossRef] [Google Scholar]
- Guidelines for Intensified Tuberculosis Case- finding and Isoniazid Preventative Therapy for People Living with HIV in Resource-constrained Settings 11592 Library Collection. Available from: https://www.stacks.cdc.gov/view/cdc/11592/Share [Last accessed on 2016 May 04]
- [Google Scholar]
- Updated National Guidelines for Pediatric Tuberculosis in India. 2012. Available from: http://www.indianpediatrics.net/mar2013/mar-301-306.htm [Last accessed on 2017 Apr 04]
- [CrossRef] [PubMed] [Google Scholar]






