Translate this page into:
Prevalence of antinuclear antibodies among healthy blood donors: An experience of a regional blood transfusion center
*Corresponding author: Santosh Kumar Sharma, Department of Life Science, School of Basic Sciences and Research, Sharda University, Greater Noida, Uttar Pradesh, India. santosh13480@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sharma N, Sharma V, Sharma SK, Thakur SK, Singh S. Prevalence of antinuclear antibodies among healthy blood donors: An experience of a regional blood transfusion center. Indian J Med Sci 2023;75:133-5.
Abstract
Objectives:
Antinuclear antibodies (ANAs) are antibodies directed against one or more molecules within the nucleus. Although ANA is present in patients suffering from connective tissue diseases, few reports reveal the presence of ANA in a healthy population. The present study aimed to identify the prevalence of ANA in healthy blood donors.
Materials and Method:
Blood samples from 370 healthy blood donors were included in the present study. To detect serum ANA, an indirect immunofluorescence technique was used using HEp-2000 slides. A titer of 1:80 was used and the type of pattern (if positive) cases were also noticed.
Results:
Out of 370 healthy donors, there were 187 males and 183 females (M: F = 1.02:1). ANA was detected in four out of 370 samples (1.081%). All the positive donors were female (100%). Among all the positive cases, three cases showed a speckled pattern and one showed a homogenous pattern at 1:80 dilution.
Conclusion:
In conclusion, there is a low prevalence of ANA positivity among healthy individuals. Although, along with clinical signs and symptoms, ANA is diagnostic of autoimmune disease, the mere presence of ANA is not synonymous with the presence of clinically significant autoimmune disease.
Keywords
Antinuclear antibody
Blood donors
HEp-2000
INTRODUCTION
Antinuclear antibodies (ANAs) are autoantibodies directed against one or more molecules within the nucleus.[1] ANA testing was first described by Hargraves et al. in 1948. They identified a cell they called an “L.E. cell.”[2] ANA is a characteristic feature of autoimmune disorders.[3] ANA identification helps in the diagnosis of connective tissue autoimmune disorders (CTD) such as Sjogren’s syndrome, systemic lupus erythematosus (SLE), and polymyositis/dermatomyositis.[4]
ANAs are antibodies that bind with the nuclear materials within the nucleus such as nucleic acids, proteins, and nucleic acid-protein complexes.[3] ANAs are classified into two groups; first, antibodies against DNA/histones, and other, against nuclear material. Antibodies against histones and DNA comprise anti-histone antibodies and anti-dsDNA antibodies, respectively. The rest of the category comprises an additional targeted nuclear antigen.[1]
The method for the detection of these autoantibodies is an indirect immunofluorescence technique using HEp-2 substrate.[5] It is considered a reference gold standard method.[6] Initially, liver and kidney sections from rats were used as substrates to detect ANAs by indirect immunofluorescence antibody (IFA) technique. In recent years, human epithelial type-2 (Hep-2) cells are used to detect ANAs by indirect IFA technique.[7-9]
ANA is present in patients suffering from CTD. However, few reports indicated the presence of autoantibodies in healthy individuals. The present study aimed to identify the prevalence of ANA in healthy blood donors.[10]
MATERIALS AND METHODS
The present study was conducted at a Regional Blood Transfusion Center associated with a tertiary care hospital and medical college located in Malka Ganj, Delhi after getting an Institutional Ethical Clearance certificate (F N0: IEC/NDMC/2021/69). Blood samples from 370 healthy blood donors were included in the present study.
Clinical history was obtained from the donors to exclude the high-risk behavior and potentially unfit conditions, followed by a general physical examination for fitness as a donor, then blood was drawn. Blood samples were also taken in plain and ethylenediaminetetraacetic acid vials for blood grouping and transfusion-transmitted infections testing. To detect serum ANA, the indirect immunofluorescence (IFA) technique was used using the ANA HEp-2000® Fluorescent Test system kit (Immunoconcepts, Germany). A titer of 1:80 was used and the type of pattern was also noticed for all positive cases. The brief protocol for ANA detection by IFA technique was as follows: 5 µL serum was mixed with 395 µL of diluent (provided with the kit) to get the final dilution of 1:80. About 30 µL of diluted serum was added in well on HEp-2000 slide. For 30 min, slides were incubated at room temperature followed by incubated slide washing with Phosphate Buffered Saline (provided with the kit). Fluorescein Isothiocyanate (FITC) conjugate (provided with the kit) was added in well and again incubated for 30 min at room temperature in dark followed by washing with phosphate-buffered saline and mounting with aqueous mounting media. Positive and negative controls were included with every slide.
RESULTS
Out of 370 healthy donors, there were 187 males and 183 females (M: F = 1.02:1). The mean age of the donors was 28.21 years (SD ± 6.06) with a range of 18–54 years. The mean age of female donors was 25.73 years (SD ± 4.21), whereas the mean age of male donors was 30.64 years (SD ± 6.59).
ANA was detected in four out of 370 samples (1.081%). All the positive donors were females (100%, [Figure 1]). Out of four positive cases, three cases showed speckled patterns and one showed homogenous pattern positivity at 1:80 dilutions [Figure 2].
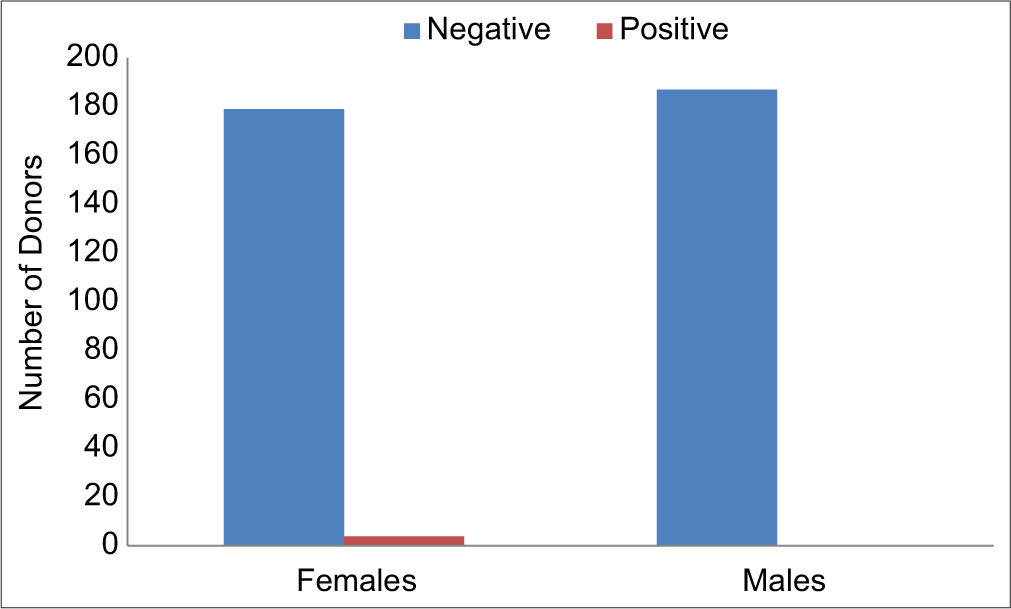
- Gender-wise distribution of donors and ANA positivity. ANA: Antinuclear antibodies.

- Homogenous pattern positivity observed in one of our donors.
DISCUSSION
Recently, there is a proposed hypothesis that the prevalence of ANA in the general population may be associated with immune disorders.[11] Few studies reported that there is a low prevalence of autoantibodies in the general population.[10]
Guo et al.,[12] conducted a study to explore the ANA positive rate among the general population of china. About 20,970 samples were taken from the physical examination center in China and also assessed the ANA positivity and its specificity by indirect immunofluorescence and line immunoassays, respectively. A total of 6800 children aged from 2 to 18 years were enlisted, including 3300 girls and 3200 boys, and 14,170 other participants (including 7220 women and 6950 men) were taken by random sampling. Overall, positivity was 5.92% in their study. The prevalence of ANA positivity was correlated with age for both sexes. They found significant differences among different age groups at 10 year of intervals except for age more than 80 years (P < 0.05). Furthermore, 1243 ANA-positive samples were analyzed with line immunoassays. In terms of the specific autoantibodies, a significant difference was seen among age groups and between sex groups (P < 0.01). Their study revealed that the females had a higher positivity rate of ANA than males (c2 = 278.55; P < 0.01). Although in our study of healthy blood donors, the positivity for ANA was 1.08%, we also found that all the positive cases were female donors.
With an objective to decrease the misdiagnosis rate of autoimmune diseases, Li et al.,[13] conducted an epidemiological study to assess the presence of ANA in a healthy population. Samples from 25,110 residents were included who received health checkups at Baoding NO.1 central hospital, China. The age of the subjects ranged from 4 months to 93 years. Using indirect immunofluorescence ANA, titers were detected. Moreover, line immunoassays detected another 15 types of ANA-specific antibodies. They found that the rate of ANA positive with titer >1:100 was 14.01%, and ANA positive rate for females was higher than for males (19.05% female, 9.04% male; P < 0.01). The rate of ANA positive with titer >1:320 was 5.93% and also the positive rate for females was higher than for males (8.68% female, 3.21% male; P < 0.01). Among all ANA positive with titer >1:320, 1489 people were detected with other ANA-specific antibodies. The study also revealed that the most common ANA-specific antibodies were anti-Ro-52 (212), followed by AMA-M2 (189) and anti-SSA (144). These positive individuals are asymptomatic.[14] These autoantibodies include anti-nuclear antigen antibodies. These individuals may remain asymptomatic for life or develop the autoimmune disease later in life. These facts are supported by the observation that, the frequency of autoimmune disease ranges from 5% to 7% in the general population,[15] whereas the frequency of SLE is a mere 0.1%.[16]
Many previous studies have concluded that most of the asymptomatic individuals with ANA are unlikely to develop autoimmune disease in the future. Hence, monitoring of the ANA titer and further workup of autoimmune diseases is not recommended for these asymptomatic individuals.[17,18]
CONCLUSION
There is low but a definite prevalence of ANA positivity among healthy individuals. Although, along with clinical signs and symptoms, ANA is diagnostic of autoimmune disease, the mere presence of ANA is not synonymous with the presence of clinically significant autoimmune disease.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Biochemistry, Antinuclear Antibodies (ANA) Treasure Island (FL): StatPearls Publishing; 2022.
- [Google Scholar]
- Presentation of two bone marrow elements; the tart cell and the L.E. cell. Proc Staff Meet Mayo Clin. 1948;23:25-8.
- [Google Scholar]
- Clinical interpretation of antinuclear antibody tests in systemic rheumatic diseases. Mod Rheumatol. 2009;19:219-28.
- [CrossRef] [PubMed] [Google Scholar]
- Pattern on the antinuclear antibody-Hep-2 test is a critical parameter for discriminating 119 antinuclear anti-body positive healthy individuals and patients with autoimmune rheumatic 120 diseases. Arthritis Rheum. 2011;63:191-200.
- [CrossRef] [PubMed] [Google Scholar]
- International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis. 2014;73:17-23.
- [CrossRef] [PubMed] [Google Scholar]
- Significance of nuclear immunofluorescent patterns. Ann Rheum Dis. 1969;28:313-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical comparison of cultured human epithelial cells and rat liver as substrates for the fluorescent antinuclear antibody test. J Rheumatol. 1985;12:265-9.
- [Google Scholar]
- The prevalence of antinuclear antibodies in healthy young persons and adults, comparing rat liver tissue sections with HEp-2 cells as antigen substrate. Clin Exp Rheumatol. 1994;12:137-41.
- [Google Scholar]
- Autoantibody profiling to identify individuals at risk for systemic lupus erythematosus. J Autoimmun. 2006;27:153-60.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic predisposition to autoimmunity-what have we learned? Semin Immunol. 2011;23:67-83.
- [CrossRef] [PubMed] [Google Scholar]
- The prevalence of antinuclear antibodies in the general population of china: A cross-sectional study. Curr Ther Res Clin Exp. 2014;76:116-9.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiological survey of antinuclear antibodies in healthy population and analysis of clinical characteristics of positive population. J Clin Lab Anal. 2019;33:e22965.
- [CrossRef] [Google Scholar]
- Antinuclear antibodies in healthy people and non-rheumatic diseases-diagnostic and clinical implications. Reumatologia. 2018;56:243-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: Estimates obtained using hospitalization data. Arthritis Rheum. 2007;56:2092-4.
- [CrossRef] [PubMed] [Google Scholar]
- The incidence of antinuclear antibodies (ANA) detected by indirect immunofluorescence assay (IFA) method. Med Arh. 2007;61:16-9.
- [Google Scholar]
- Risk factors for ANA positivity in healthy persons. Arthritis Res Ther. 2011;13:R38.
- [CrossRef] [PubMed] [Google Scholar]







