Translate this page into:
A deep dive into chickenpox epidemiology and outbreaks: A retrospective study in a tribal-dominated district of Western India
*Corresponding author: Vikram Khan, Integrated Disease Surveillance Program, Directorate of Medical and Health Services, Silvassa, India. khandst@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Khan V, Sanghai AA, Zala D, Babariya MJ, Das V. A deep dive into chickenpox epidemiology and outbreaks: A retrospective study in a tribal-dominated district of Western India. Indian J Med Sci. 2024;76:36-42. doi: 10.25259/IJMS_196_2023
Abstract
Objectives:
This retrospective observational study conducted in the district of Dadra and Nagar Haveli, Western India, over the past 9 years aimed to comprehensively investigate the epidemiology of chickenpox. The objectives were to analyze demographic and clinical characteristics, examine temporal trends, identify outbreak locations, assess outbreak intensity and duration, determine laboratory-confirmed cases, and provide insights for public health interventions.
Materials and Methods:
The study employed a retrospective approach, gathering data on chickenpox cases in the region. Demographic information, clinical profiles, and outbreak details were analyzed. Temporal variations and seasonal trends were assessed. Laboratory confirmation was achieved through serology and molecular methods. Locations of outbreaks were identified, and their characteristics were evaluated.
Results:
The study revealed that the 6–10 age groups were most vulnerable, with mild-to-moderate symptoms predominantly observed. Significant variations in cases occurred year round, with peaks between November and February. Out of 25 recorded outbreaks and three early warning signals, Aganwadis, play schools, and schools were common outbreak locations. Most outbreaks were low intensity, and laboratory confirmation identified Clade-1 Varicella-Zoster virus as the causative agent.
Conclusion:
These findings provide essential insights for public health officials. Identifying the vulnerable age group and high-risk locations allows for targeted vaccination campaigns. Moreover, the study underscores the need for continuous monitoring and surveillance to detect outbreaks early and mitigate their spread effectively, emphasizing the importance of proactive prevention and control measures for chickenpox in the region.
Keywords
Varicella
Outbreak
Epidemiology
Risk factors
INTRODUCTION
Chickenpox is a contagious viral infection caused by the Varicella-Zoster virus (VZV). It spreads through the air or direct contact with blisters.[1] The diseases can affect people of all ages, genders, and ethnicities.[2] However, it is commonly seen in children under the age of 10 years.[3] The symptoms of chickenpox are usually mild but can lead to serious complications, especially in people with weakened immune systems or pregnant women.[4] The most serious complications from chickenpox include pneumonia, encephalitis (brain inflammation), and sepsis (a severe infection of the bloodstream).[1] The overall mortality rate for chickenpox is low, but the disease is still responsible for a significant number of hospitalizations and deaths in unvaccinated populations.[1,2,5] Potential transmission of chickenpox is believed to be affected by climate, and some studies have examined the relationship between weather variability and the incidence of infectious diseases.[6-8] The exact timing of chickenpox transmission can vary from year to year and from region to region, but the disease is generally more common during the colder and spring months.[3,9] Regardless of climate, the timing of chickenpox transmission is influenced by several factors, including vaccination coverage, population density, and the presence of susceptible individuals.[4,10] Vaccination is the sole preventative measure for chickenpox and is readily available for children.[10,11] Developed nations have observed a decline in chickenpox cases due to vaccination while developing nations remain at risk since the vaccine is optional in countries like India. Without vaccination, there is a gradual rise in seroprevalence with age, peaking in adolescents and adults.[2,12] Several outbreaks of chickenpox have been reported in different parts of the world and India from time to time.[13,14] These outbreaks highlight the importance of continued monitoring and surveillance of chickenpox to detect outbreaks early and prevent their spread.
The study explores chickenpox outbreaks and epidemiology in Dadra and Nagar Haveli (D&NH), a district in Western India with a large tribal population spanning the last nine years. The goal is to gather essential data on the disease’s spread, enabling the development of effective public health strategies to reduce chickenpox incidence and associated complications.
MATERIALS AND METHODS
Study site
It is an observational retrospective study carried out in the D&NH district of the Union Territory of D&NH and Daman and Diu (DD) situated at the Western Ghats of India (latitude, 20° 54’ 41” N to 20° 21’ 36” N and longitude - 72° 54’ 41” N to 73° 13’ 13” N). The 487 sq km area is forested hills, occupied by mainly tribes (population 4.5 lakh) in 72 villages and one town. Health services to natives and migrant populations are provided through seventy-one Sub Centers/Health and Wellness Centers, Nine Primary Health Centers (PHC), Two Community Health Centers, one 100 bedded sub district hospitals in rural areas and one 650 bedded district hospital at district headquarter. In addition, in the private sector, there are ten private hospitals and more than 100 medical practitioners (allopathic, ayush, dental, and homeopathic). In D&NH large-scale development, urbanization, and industrialization have come up in the recent past. Due to the subsidiary taxes, many large (20), medium (564), and small (2118) industries have been established. Approximately 2.5 lakh skilled and unskilled workforce come to D&NH from different states of India are working in these units.
Surveillance mechanism
Under the Integrated Diseases Surveillance Program (IDSP), the portal-based, three-tier surveillance of chickenpox has been in place since 2009. This system involves both active and passive surveillance methods to track the incidence of chickenpox in a given population. Active surveillance is carried out by grassroot level workers who conduct house-to-house visits and record information about patients with symptoms of chickenpox in a portal. In addition, clinicians are involved in presumptive surveillance and record information about patients who are clinically suspected of having chickenpox and visit their dispensaries. For laboratory confirmation of chickenpox cases in the IDSP, the clinical specimens collected were sent to the referral laboratory, which was identified at the National Institute of Virology in Pune, Maharashtra. At the referral laboratory, the samples were diagnosed using various methods such as enzyme-linked immunosorbent assay, polymerase chain reaction (PCR), and sequencing.
Case definition
During the chickenpox surveillance under the IDSP, the case definition stated that a case of chickenpox was considered to be an “acute illness with diffuse (generalized) macular/papular/vesicular rash, and epidemiologic linkage to another probable or confirmed case, or laboratory confirmation by VZV-specific immunoglobulin M antibodies detection or VZV DNA detection by PCR or isolation of chickenpox virus from a clinical specimen.” In the IDSP for chickenpox, the medical officers working at the PHC were responsible for clinically diagnosing all suspected cases. They collected clinical specimens such as serum, blister/skin swabs, urine, and throat swabs from the cases for laboratory diagnosis. The same case definition was used by both the medical officers at the PHC and the rapid response team during outbreaks or periods of early warning.
Data source and variable
The different data sources were used to provide a comprehensive overview of the research methodology, as described in Table 1. Demographic, health infrastructure and population data were collected either from the district data center or census year 2011. Epidemiological variables were derived from the state surveillance unit of IDSP or district data center. The data were analyzed using Microsoft Excel, 2010 software. Descriptive statistics such as frequency and proportion were used to analyze the demographic factors and their outcome.
| Particulars | Sources of information | Methodology | Variables |
|---|---|---|---|
| Geographical information of the study site | District data center | Descriptive analysis |
|
| Variables of total cases of chickenpox | SSU, IDSP/State data center | Descriptive analysis |
|
| Variables of outbreaks/EWS | SSU, IDSP/State data center | Descriptive analysis |
|
SSU: State surveillance unit, IDSP: Integrated disease surveillance project/State data center, GIS: Geographic information system, EWS: Early warning signal
RESULTS
Between 2014 and 2022, 2554 clinical chickenpox cases were reported in the D&NH district. Of these, 31.87% (814 cases) were reported during outbreaks, with the highest number of cases reported in the urban area (37.71%), followed by the tribal areas (28.94%), semi-urban/industry areas, and outof-district areas (9.95% each). A geographical distribution of the cases can be seen in Figure 1. The most vulnerable age group for chickenpox was 6–10 years old (27.74%), followed by 1–5 years old (17.08%), and 11–15 years old (15.74%). Males were outnumbered by females. Most cases (97.26%) had mild-to-moderate symptoms, not needing hospitalization. Only 2.74% were severe, requiring hospital care. Cases varied widely annually: highest in 2017 (538) and lowest in 2014 (156). Most cases occurred from February to November (50.23%), with a peak in January 2019 (232 cases). The fewest cases were in June 2022 (7). Majority were reported between March–June (32.93%), and July–October (16.84%) [Table 2].
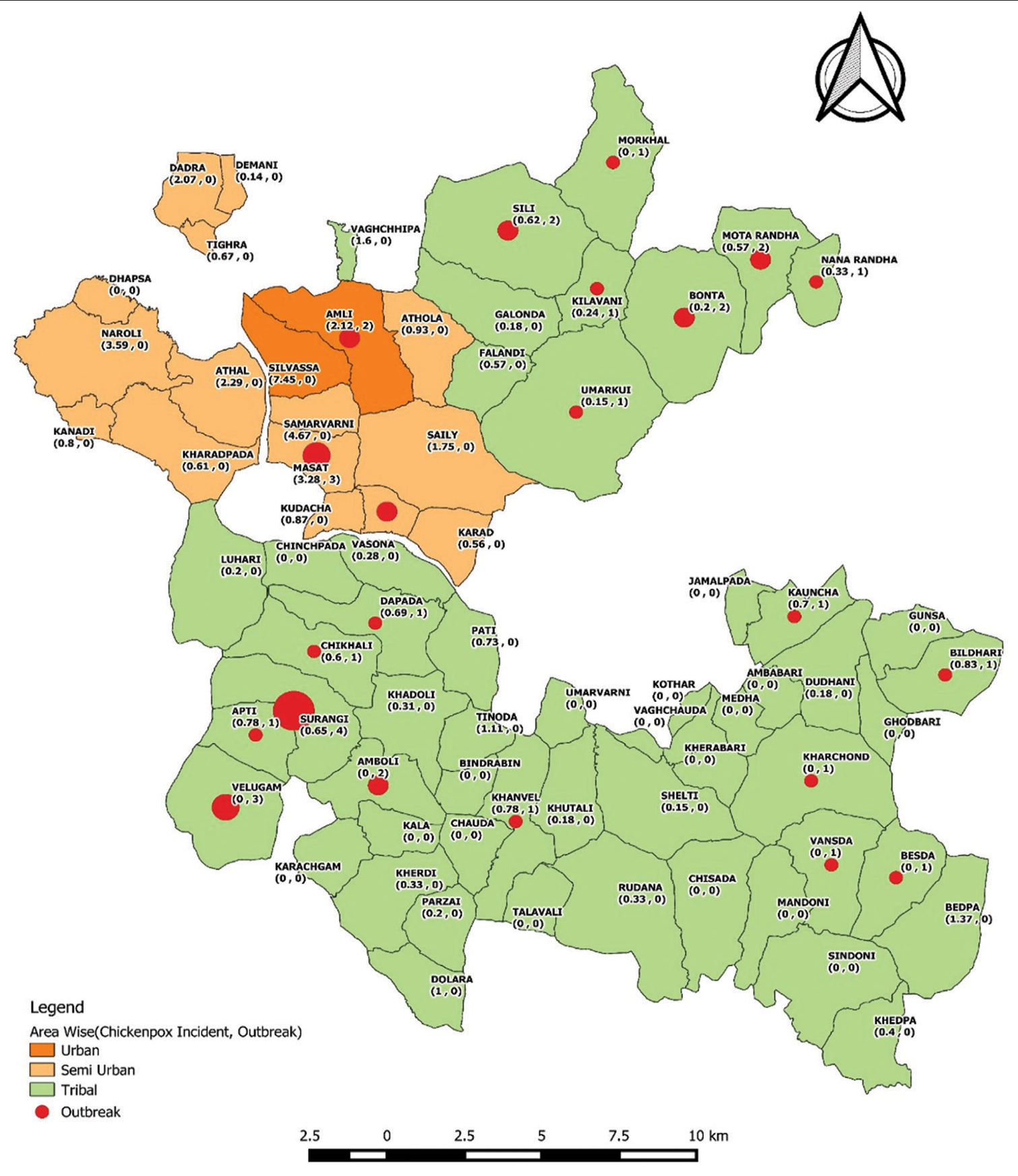
- The spatial distribution of reported cases of chickenpox and outbreaks from 2014 to 2022.
| Variables | n(%) |
|---|---|
| Total number of cases | 2554 |
| Number of cases reported during the outbreak | 814 (31.87) |
| Geographical distribution of cases | |
| Tribal | 739 (28.94) |
| Semi-urban/industry | 598 (23.41) |
| Urban | 963 (37.71) |
| Out of district | 254 (9.95) |
| Seasonality of cases | |
| November to February | 1283 (50.23) |
| March to June | 841 (32.93) |
| July to October | 430 (16.84) |
| Yearly distribution | Total |
| 2014 | 156 (6.11) |
| 2015 | 197 (7.71) |
| 2016 | 459 (17.97) |
| 2017 | 538 (21.06) |
| 2018 | 226 (8.85) |
| 2019 | 530 (20.75) |
| 2020 | 211 (8.26) |
| 2021 | 32 (1.25) |
| 2022 | 205 (8.03) |
| Sex wise distribution | Total cases |
| Male | 1463 (57.28) |
| Female | 1091 (42.72) |
| Age wise distribution | Total |
| <1 | 22 (0.87) |
| 1–5 | 436 (17.08) |
| 6–10 | 709 (27.74) |
| 11–15 | 402 (15.74) |
| 16–20 | 268 (10.51) |
| 21–25 | 259 (10.14) |
| 26–30 | 231 (9.05) |
| Above 31 | 227 (8.89) |
| Hospitalization status | |
| Outdoor patients | 2484 (97.26) |
| Indoor patients | 70 (2.74) |
| Type of OPD | |
| Casualty | 15 (0.86) |
| Dermatology | 846 (48.32) |
| General/medicine | 652 (37.24) |
| Neonatal/pediatric | 238 (13.59) |
| Reporting institutions | |
| Health and wellness centers/sub-center | 361 (14.13) |
| Primary health centers | 301 (11.79) |
| Community health centers | 5 (0.20) |
| Sub-district hospital | 3 (0.12) |
| District hospital | 1845 (72.24) |
| Other | 39 (1.53) |
OPD: Outword patient department, n: Total number
During the past 9 years, 25 outbreaks and 3 early warning signals of chickenpox have been recorded in the district. The majority of events were reported from November to February, followed by March to June and July to October. The highest number of outbreaks occurred in tribal areas, then semi-urban/industrial areas, and lastly, urban areas. The most common locations for outbreaks and early warning signals were anganwadis/play schools/schools (17 events, 60.71%) followed by chawls/slums and communities (11 events, 39.29%). During the outbreaks, 60.71% had low intensity (attack rates <0.5), 17.86% had rates between 0.51% and 1.0%, 14.29% had rates between 1.01% and 1.5%, and 7.14% had rates above 1.5%. The most susceptible age group was 6–10 years, followed by 1–5 years, 11–15 years, 16–20 years, and those over 20 years old. The duration of chickenpox outbreaks varied widely, with 11 events (39.29%) lasting for over 10 weeks and 2 events (7.14%) lasting only 1 or 2 weeks. The remaining outbreaks lasted between 3 and 4 weeks (7 events or 25.00%), 5–6 weeks (4 events or 14.29%), 7–8 weeks (3 events or 10.71%), and 9–10 weeks (1 event or 3.57%) [Table 3]. The samples of 11 outbreaks (39.29%) were sent to the referral laboratory for laboratory confirmation. Furthermore, the results confirmed the presence of the clade-1 VZV as the causative organism in the district.
| Sr. no. | Year | Area | Total cases | Incidence rate/1000 population | Epicenter | Whether confirm by laboratory | Attack rate | MF ratio | Most affected age group | Length of event (Weeks) | Month |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OB 1 | 2014 | Tribal | 75 | 12.20 | Anganwadi and School | No | 1.22 | 1.08 | 6–10 years | 4 | Dec |
| OB 2 | 2015 | Tribal | 23 | 8.00 | Anganwadi and School | No | 0.80 | 1.30 | 1–5 years | 3 | Mar |
| OB 3 | 2016 | Tribal | 25 | 11.11 | Anganwadi | No | 1.11 | 1.08 | 6–10 years | 8 | Jan |
| OB 4 | 2016 | Tribal | 18 | 4.70 | Anganwadi and School | No | 0.47 | 2.00 | 6–10 years | 10 | Feb |
| OB 5 | 2016 | Tribal | 15 | 7.00 | Anganwadi and School | No | 0.70 | 0.29 | 11–15 years | 5 | Feb |
| OB 6 | 2016 | Semi-urban | 13 | 0.99 | School | No | 0.10 | 0.86 | 11–15 years | 6 | Apr |
| OB 7 | 2016 | Tribal | 9 | 1.63 | Community | No | 0.16 | 3.00 | 1–5 years | 2 | Mar |
| OB 8 | 2016 | Semi-urban | 80 | 8.62 | Anganwadi and School | Yes | 0.86 | 1.05 | 1–5 years | 14 | Dec |
| OB 9 | 2016 | Tribal | 11 | 2.47 | Community | No | 0.25 | 0.83 | 6–10 years | 4 | Mar |
| OB 10 | 2016 | Tribal | 69 | 12.36 | Industry | Yes | 1.24 | 3.93 | 15 years above | 13 | Dec |
| OB 11 | 2016 | Urban | 19 | 1.19 | Chawl | No | 0.12 | 0.73 | 21–25 years | 5 | Feb |
| EWS 1 | 2016 | Tribal | 11 | 2.20 | Community | No | 0.22 | 1.75 | 6–10 years | 3 | Jun |
| EWS 2 | 2016 | Semi-urban | 7 | 0.86 | Community | Yes | 0.09 | 1.33 | 1–5 years | 3 | Dec |
| OB 12 | 2017 | Semi-urban | 21 | 1.61 | Chawl | Yes | 0.16 | 0.40 | 6–10 years | 2 | Feb |
| OB 13 | 2018 | Tribal | 12 | 4.72 | School | Yes | 0.47 | 1.00 | 6–10 years | 6 | Dec |
| OB 14 | 2018 | Tribal | 27 | 1.39 | School | Yes | 0.14 | 3.50 | 11–15 years | 13 | Feb |
| OB 15 | 2019 | Tribal | 13 | 2.42 | Community | No | 0.24 | 0.44 | 6–10 years | 13 | Jan |
| OB 16 | 2019 | Tribal | 34 | 11.93 | Anganwadi | Yes | 1.19 | 1.00 | 1–5 years | 8 | Jan |
| OB 17 | 2019 | Urban | 13 | 1.28 | Anganwadi and School | No | 0.13 | 0.44 | 1–5 years | 15 | Jan |
| OB 18 | 2019 | Tribal | 9 | 0.99 | Anganwadi and School | No | 0.10 | 0.80 | 6–10 years | 5 | Mar |
| OB 19 | 2019 | Tribal | 63 | 3.55 | School | Yes | 0.35 | 1.10 | 6–10 years | 17 | Jan |
| OB 20 | 2019 | Tribal | 19 | 3.47 | Community | No | 0.35 | 0.90 | 6–10 year | 8 | Apr |
| OB 21 | 2019 | Tribal | 79 | 15.20 | School | Yes | 1.52 | 2.43 | 6–10 years | 15 | Jan |
| EWS 3 | 2019 | Tribal | 7 | 3.77 | Community | No | 0.38 | 1.33 | 6–10 years | 3 | Dec |
| OB 22 | 2020 | Tribal | 16 | 5.91 | Anganwadi | No | 0.59 | 1.29 | 1–5 years | 6 | Jan |
| OB 23 | 2020 | Tribal | 64 | 16.87 | School | No | 1.69 | 0.83 | 6–10 years | 3 | Jan |
| OB 24 | 2022 | Tribal | 23 | 4.94 | Industry chawl | Yes | 0.49 | 15 years above | 5 | Feb | |
| OB 25 | 2022 | Tribal | 39 | 6.45 | School hostel | Yes | 0.64 | 2.55 | 15 years above | 6 | Jul |
OB: Outbreak, EWS: Early warning signal
DISCUSSION
The present study is an observational retrospective study conducted in the district of D&NH of Western India. The study aims to investigate the epidemiology of chickenpox and its outbreaks in the district over the past 9 years. The current study’s findings indicate a variation in the incidence rates of chickenpox according to age, with the highest rates being observed among children aged 6–10 years. This age group has been identified as particularly susceptible to chickenpox, consistent with previous studies conducted in Iran, China, and the USA.[9,15,16] The higher incidence of chickenpox in certain age groups may be due to a combination of factors such as higher exposure to the virus, weaker immune systems, and variations in vaccination coverage.[3] Furthermore, our study found that the majority of cases were mild-to-moderate and did not require hospitalization, which is consistent with the findings of previous studies.[13] The highest number of cases and outbreaks occurred in the winter and spring months (January–April), and the lowest number of cases occurred in the summer and fall months (June–November). This is consistent with previous studies conducted in different regions of the world, including Taiwan,[7] China[8], and the United States.[9] One possible explanation for this seasonal variation is the impact of temperature and humidity on the survival of the varicella virus. The virus is known to survive longer in cooler and less humid conditions, which may explain why chickenpox is higher during the colder months.[3] In addition, the winter months are associated with decreased sunlight exposure, which may lead to lower levels of Vitamin D and a weakened immune system, making individuals more susceptible to infections like chickenpox.[17] In addition, close proximity in the house due to cold weather and school schedules may also influence the incidence of chickenpox, as children are more likely to come into close contact with each other during the winter and spring months.[2,13,16] The higher number of reported chickenpox cases in urban areas, compared to semi-urban and tribal areas, could be attributed to several factors, including population density, better reporting systems, and high migration. Yang et al. conducted a study in China, which found that higher population density was significantly associated with an increased risk of chickenpox incidence.[8] Moreover, urban areas may have better health-care facilities and reporting systems, leading to higher detection and reporting of cases. Chickenpox incidence has been found to be slightly higher in males than in females, which could be due to a combination of biological and behavioral factors, as male children were at a higher risk of developing chickenpox than female children, possibly due to a greater tendency for boys to engage in outdoor activities and have more close contact with other children. The study highlights the epi-centers of chickenpox outbreaks in different settings. The results suggest that outbreaks were reported in four different types of settings: anganwadi/play school, school, industry chawl, and community/slum. These settings are known to have high population density and close contact between individuals, which may facilitate the spread of the disease.
The present study calculated the attack rate for various events during the outbreak of chickenpox. Results showed that 60.71% of events had an attack rate of <0.5, 17.86% had an attack rate between 0.51% and 1.0%, 14.29% had an attack rate between 1.01% and 1.5%, and 7.14% had an attack rate > 1.5. The low attack rate in majority of events suggests that appropriate preventive measures have effectively controlled the outbreak. However, the higher attack rate in few events highlights the need for targeted interventions, such as vaccination campaigns, isolation and quarantine of infected individuals, and health education programs to prevent further disease transmission. Previous studies have also reported on attack rates during chickenpox outbreaks. For instance, a study conducted in rural India found an attack rate of 7.4%, which was higher than the attack rate observed in the present study.[14] Conversely, a study conducted in a school in Taiwan reported an attack rate of 1.3%, which was similar to the attack rate observed in the present study.[7] The present study analyzed the duration of 28 outbreak events and found that 25.00% of outbreaks lasted more than 10 weeks, while 7.14% lasted 1–2 weeks. The varying durations of outbreaks are consistent with previous studies. A study conducted in a school in Taiwan reported outbreaks that lasted for 3–6 weeks, while another study in primary schools in China reported an outbreak that lasted for 10 weeks.[7,16] The duration of outbreaks can serve as an indicator of the effectiveness of control measures implemented. Shorter durations of outbreaks suggest that prompt control measures were implemented, while longer durations may indicate a failure in control measures or a delay in their implementation. According to a study conducted between 2000 and 2006, the death rate from chickenpox was reported to be 0.26/1 million population, with the majority of deaths occurring in unvaccinated individuals or those with unknown vaccination status.[3] In contrast, the present study did not encounter any deaths related to chickenpox. In this study, 97.26% of chickenpox cases were treated on an outpatient basis, while 2.74% required indoor admission for treatment. This finding is consistent with previous studies that have reported similar distributions of hospitalization status in chickenpox cases.[18] The confirmation of chickenpox outbreaks through laboratory diagnosis is essential for the appropriate management of the disease and the implementation of control measures. In this study, 39.29% of outbreak samples were sent to a referral laboratory for confirmation, and all of them were confirmed using both serological and molecular methods. The detection of the clade-1 VZV strain in D&NH is consistent with prior research,[19,20] emphasizing the importance of ongoing monitoring and surveillance of VZV genotypes, particularly in areas with high incidences of chickenpox outbreaks.
CONCLUSION
The study was conducted in D&NH, India, spanning 9 years, revealed a significant chickenpox burden. Children aged 6–11 were most vulnerable. Cases were mostly mild, peaking in winter and spring. Clade-1 VZV was identified as the cause. While prevention measures helped, targeted actions like vaccinations are vital to curb future outbreaks.
Acknowledgment
The authors would like to thank the Director of Medical and Health Services, UT of DNH&DD, and Mission Director National Health Mission, UT of DNH&DD, for their support during the study.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Chickenpox (Varicella) 2022. Available from: https://www.cdc.gov/chickenpox/about/index.html [Last accessed on 2023 Sep 18]
- [Google Scholar]
- Epidemiology and prevention of vaccine-preventable diseases (14th ed). Washington, D.C: Centers for Disease Control and Prevention, Public Health Foundation; 2021.
- [Google Scholar]
- Changing varicella epidemiology in active surveillance sites--United States, 1995-2005. J Infect Dis. 2008;197(Suppl 2):S71-5.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome of chicken pox in adult immunocompetent patients. J Pak Assoc Dermatol. 2006;16:141-8.
- [Google Scholar]
- International travel and health. Available from: https://www.who.int/publications/i/item/9789241580472 [Last accessed on 2022 Jun 18]
- [Google Scholar]
- Role of meteorological conditions in reported chickenpox cases in Wuhan and Hong Kong, China. BMC Infect Dis. 2017;17:538.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for chickenpox incidence in Taiwan from a large-scale computerized database. Int J Dermatol. 2007;46:362-6.
- [CrossRef] [PubMed] [Google Scholar]
- Association between the incidence of varicella and meteorological conditions in Jinan, Eastern China, 2012-2014. BMC Infect Dis. 2016;16:179.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of varicella hospitalizations in the United States, 1995-2005. J Infect Dis. 2008;197(Suppl 2):S120-6.
- [CrossRef] [PubMed] [Google Scholar]
- Controversies in chickenpox immunization. Indian J Pediatr. 2003;70:503-7.
- [CrossRef] [PubMed] [Google Scholar]
- Digital epidemiology reveals global childhood disease seasonality and the effects of immunization. Proc Natl Acad Sci U S A. 2016;113:6689-94.
- [CrossRef] [PubMed] [Google Scholar]
- Review of varicella zoster seroepidemiology in India and Southeast Asia. Trop Med Int Health. 1998;3:886-90.
- [CrossRef] [PubMed] [Google Scholar]
- Chickenpox outbreak in a highly vaccinated school population. Pediatrics. 2004;113:455-9.
- [CrossRef] [PubMed] [Google Scholar]
- An epidemiological study of outbreak investigation of chickenpox in remote hamlets of a tribal state in India. Cureus. 2022;14:e26454.
- [CrossRef] [Google Scholar]
- Seroprevalence and risk factors of varicella zoster infection in Iranian adolescents: A multilevel analysis; the CASPIAN-III study. PLoS One. 2016;11:e0158398.
- [CrossRef] [PubMed] [Google Scholar]
- A persistent outbreak of varicella in a primary school in Dongguan City, Guangdong Province, China. J Int Med Res. 2020;48:1-12.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal Vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr. 2009;139:1157-61.
- [CrossRef] [PubMed] [Google Scholar]
- Prevention of varicella: Recommendations of the advisory committee on immunization practices (ACIP) MMWR Recomm Rep. 2007;57:1-40.
- [Google Scholar]
- Varicella outbreak in children from Silvassa, Dadra and Nagar Haveli, India. Indian Pediatr. 2021;58:483-4.
- [CrossRef] [Google Scholar]
- Chickenpox outbreak in a tribal and industrial zone from the Union Territory of Dadra and Nagar Haveli, India. Epidemiol Infect. 2018;146:476-80.
- [CrossRef] [PubMed] [Google Scholar]






