Translate this page into:
A declining trend of hepatitis A and hepatitis E at tertiary care hospital in South Gujarat
*Corresponding author: Swati Sugnesh Patel, Department of Community Medicine, Surat Municipal Institute of Medical Education and Research, Surat, Gujarat, India. swati84patel@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gandhi TN, Patel SS, Chaudhary A, Nakrani K. A declining trend of hepatitis A and hepatitis E at tertiary care hospital in South Gujarat. Indian J Med Sci. doi: 10.25259/IJMS_225_2023
Abstract
Objectives:
Hepatitis A virus (HAV) and Hepatitis E virus (HEV) both are spread through the fecal-oral route and cause acute viral hepatitis (AVH) and pose a major public health problem in India. This study was done to find out the proportion of positivity of HAV and HEV in patients with AVH and its seasonal trend.
Materials and Methods:
A retrospective study was carried out at Surat Municipal Institute of Medical Education and Research Medical College, Department of Microbiology, Surat, Gujarat. Result of 3615 blood samples of suspected AVH patients of the past 5 years (January 2018–December 2022) were taken from hospital data records. The enzyme-linked immunosorbent assay method was used to test serum samples for immunoglobulin M (IgM) HAV and IgM HEV antibodies for HAV and HEV, respectively. All samples were evaluated for liver function as well.
Results:
The positivity of HAV and HEV was 15.13% and 10.26%, respectively. The coinfection rate was 2.07%. HAV and HEV both affected males more than females. Among pregnant females, HEV infection had more positivity (6.77%) than HAV, which had 1.08% positivity. HAV and HEV infections had a seasonal trend, with the highest infection rate in the monsoon.
Conclusion:
The declining trend of cases of HAV and HEV was found in Surat city of south Gujarat which indicates increased awareness about hepatitis among people and better public health management by the civic authorities.
Keywords
Acute viral hepatitis
Hepatitis A virus
Hepatitis E virus
Pregnant women
Liver function test
Seasonal variation
Decline cases of HAV and HEV
INTRODUCTION
Hepatitis A and Hepatitis E pose significant public health challenges, leading to substantial rates of mortality and morbidity. These viruses are primarily widespread in developing countries. Both of these viruses are predominantly transmitted through the fecal-oral route and cause infections ranging from asymptomatic infection to acute viral hepatitis (AVH) of varying severity.[1-3]
Epidemiology of hepatitis A virus (HAV) is influenced by the socioeconomic status of the population, which itself is a major determinant of hygiene, water quality, and sanitation. Over the past few years, worldwide, the seroprevalence of HAV has been in decline in both developed as well as in developing countries. India has been experiencing significant socioeconomic growth over the past few decades and data also reflect this trend with a decline in HAV seroprevalence in India.[3-8]
The infection can occur as outbreaks or as sporadic cases. Outbreaks often occur during the rainy season when the chances of contamination of drinking water with sewage increase. The risk for person-to-person transmission is small, as is evident from low secondary attack rates among family members and other close contacts of those affected.[9] Hepatitis E virus (HEV) infection and disease are highly endemic in India, with nearly 60% of blood donors having circulating anti-HEV immunoglobulin G antibodies, demonstrating prior exposure to the virus.[10,11]
Till date, there has been no case-based surveillance for these viral diseases in India and the mode of surveillance has been outbreak-oriented.[3,12] Very few studies published in India indicate the long-term trend and burden of these two infectious diseases, though it is important to study the real scenario of long-term trends of HAV and HEV infections in India considering the changes in diverse socioeconomic conditions, demographic factors, and sanitation infrastructure. The present study was planned to assess the infection rate of HAV, HEV, and their coinfections in AVH cases in our hospital set up with the following objectives: (i) To study the yearly and monthly trend of HAV and HEV infection for 5 years and also determine the proportion of HAV and HEV infections and coinfections in the samples of clinically suspected patients, (ii) to evaluate the burden of HEV infection among pregnant women with hepatitis, and (iii) to find out the derangements of liver function.
MATERIALS AND METHODS
This retrospective observational study was done over 5 years from January 2018 to December 2022 at the Virology laboratory, Microbiology Department, and tertiary Hospital of Surat City which is also the center for Integrated Health Information Platform. This study included a total of 3615 inpatient department/outpatient department-based clinically suspected patient’s serum samples having clinical features of AVH such as fatigue, loss of appetite, nausea, vomiting, jaundice, and increased liver enzyme. The samples were received for serological testing against hepatitis A and hepatitis E in the institute’s Microbiology laboratory during the study period. This retrospective study was started after the approval from the Institutional Ethics Committee of Surat Municipal Institute of Medical Education and Research with Reference to ethical number (IEC 100–8/02/2022).
3–5 mL of venous blood sample of patients were collected who were clinically suspected of HAV and referred for test by the clinician. It was allowed to clot and samples were centrifuged at 3000 rpm for 10 min. Serum was separated and processed and analyzed for immunoglobulin M (IgM) antibody to HAV and HEV using IgM capture enzyme-linked immunosorbent assay kits (QUALISA Test, Biotech) in accordance with the manufacturer’s instructions. The remaining serum samples were stored in sterile screw-capped vials and preserved at −20°C. The demographic and clinical variables including age and sex were noted. Biochemical parameters such as liver function tests consisting of total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) levels were also determined.
Data analysis
Descriptive statistics was used to summarize the total number of cases per month and per year as frequency and percentage. Z-test of proportion was used to compare the proportion of positivity of HAV, HEV, and coinfection among the gender as well as pregnant and non-pregnant women considering the 95% level of significance. Graphical representation was conducted using Microsoft Excel, while the Z-test of proportion was performed using EPI tools software.
RESULTS
This study used a total of 3615 serum samples of clinically suspected cases of AVH of the past 5 years from January 2018 to December 2022. Out of them 547 (15.13%) were HAV-positive, 371 (10.26%) were HEV-positive, and 75 (2.07%) were coinfection-positive (HAV and HEV both positive). In the year 2018 out of the total samples tested, 202 (1.76%) were found to be HAV-positive, 145 (12.61%) were HEV-positive, and 25 (2.18%) were observed to be coinfected. The decrease in inflow of samples over the 5 years was 50.21% whereas the positivity of HAV was decreased by 72.8%, HEV was decreased by 64.5%, and coinfection was decreased by 68% [Figure 1a and b]. The yearly decline trend of samples and positivity of HAV and HEV can be observed over the 5 years.

- Yearly cases of hepatitis A virus, hepatitis E virus, and coinfection and their positivity. (a) Total sample tested and positivity rate and (b) yearly number of positive cases. HAV: Hepatitis A virus, HEV: Hepatitis E virus.
The year with the most positive cases was found in 2018, and there was a seasonal increase in cases throughout July, August, and September [Figure 2]. The monthly positive cases trend peaked during the monsoon (June–September). The trends of positive cases of HAV and HEV indicate the gradual decline of the cases over the period [Figure 2]. Over 5 years, the percentage of difference observed was 74% for HAV cases and 73% for HEV cases.
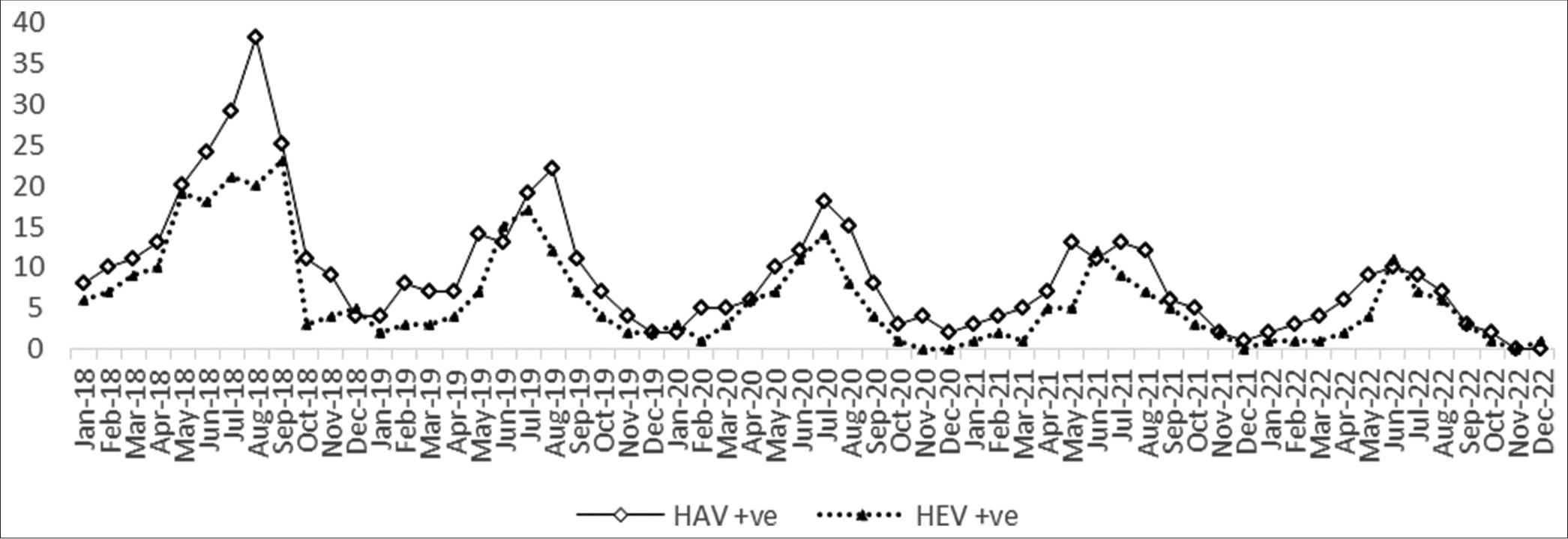
- Seasonal trend of hepatitis A virus and hepatitis E virus-positive cases. HAV: Hepatitis A virus, HEV: Hepatitis E virus.
The high proportion of males was observed in the positivity of HAV (26.6%), HEV (17.4%), and coinfection (2.8%) over 5 years. It is statistically significant [Table 1]. The proportion of HEV positivity was high among the pregnant (6.77%) whereas the proportion of HAV positivity was high among non-pregnant women (14.15%). No significant difference was observed between the proportion of coinfection present [Table 2]. Total bilirubin, serum glutamic oxaloacetic transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), and ALP were high in coinfected patients as compared to isolated HAV and HEV cases [Table 3].
| Male (n=1446) (%) | Female (n=2169) (%) | P-value | Difference (%) | 95% CI of difference | |
|---|---|---|---|---|---|
| HAV+ve | 384 (26.6) | 163 (7.5) | P<0.001 | 19.1 | (16.7–21.4) |
| HEV+ve | 252 (17.4) | 119 (5.5) | P<0.001 | 11.9 | (9.9–13.9) |
| Coinfection+ve | 40 (2.8) | 35 (1.6) | 0.013 | 1.2 | (0.025–2.2) |
HAV: Hepatitis A virus, HEV: Hepatitis E virus, CI: Confidence interval
| Pregnant (n=1300) (%) | Non-pregnant (n=869) (%) | Total | P-value | Difference (%) | 95% CI of difference | |
|---|---|---|---|---|---|---|
| HAV+ve | 40 (1.08) | 123 (14.15) | 163 | P<0.001 | 13.07 | (11.05–15.09) |
| HEV+ve | 88 (6.77) | 31 (3.57) | 119 | 0.0018 | 3.1 | (1.2–5.1) |
| Coinfection+ve | 17 (1.31) | 18 (2.07) | 35 | 0.1482 | 0.08 | (−0.03–1.9) |
HAV: Hepatitis A virus, HEV: Hepatitis E virus, CI: Confidence interval
| Biochemical tests | HAV (n=547) n(%) | HEV (n=371) n(%) | Coinfection (n=75) n(%) |
|---|---|---|---|
| Total bilirubin | 382 (69.84) | 289 (77.90) | 65 (86.67) |
| SGOT | 346 (63.25) | 270 (72.76) | 67 (89.33) |
| SGPT | 340 (62.16) | 260 (70.08) | 65 (86.87) |
| ALP | 397 (72.58) | 297 (80.05) | 75 (100) |
HAV: Hepatitis A virus, HEV: Hepatitis E virus, SGOT: Serum glutamic oxaloacetic transaminase, SGPT: Serum glutamate pyruvate transaminase, ALP: Alkaline phosphatase
DISCUSSION
In the present retrospective study, in which data from a period of 5 years were considered indicates the year-wise as well as the seasonal declining trend of infections caused by HAV, HEV, and coinfection in a tertiary care hospital in south Gujarat. A seasonal peak was observed in our study during the monsoon season, which runs from June to October which is similar to the other studies in India.[1-4,11,13,14] Understanding that there is a seasonal surge of cases in these months can help restrict the incidence through a concerted effort by the public health department before and during the monsoon season each year. Because they are frequently the initial point of contact with cases in the local community, the primary and secondary health-care systems can be an important part of this strategy.[3] A teaching hospital-based study conducted a study in Northern India for 8 years indicates the changing trend of HAV and HEV infectious cases according to seasons but a yearly increasing trend was observed in both infectious cases. The continuous decline trend of infectious cases is due to the proper sewage disposal, sanitation systems, and facility of uncontaminated drinking water sources facility provided by the municipal corporation of Surat City in south Gujarat. This trend might be the result of the implementation of the National Viral Control program launched by Ministry of Health and Family Welfare in July 2018. This program in collaboration with other national programs such as the Swachh Bharat Mission, Safe Drinking Water, and Sanitation program seems to have provided the intended result.[15]
The high proportion of males was observed in the positivity of HAV (26.6%) and HEV (17.4%), which is similar to other studies. Males predominated among both HAV and HEV cases (P < 0.01).[13-21] The proportion of coinfection in this study was reported (2.8%) which is similar to reports by other studies.[18-19]
Pregnant females were 36.96 of the total tested samples. This study shows that the proportion of HEV cases among pregnant females was high which is comparable with data from a previous study from India.[3,22-24] In developing nations, HEV infection is one of the most common causes of problems connected to pregnancy.[25] Preterm birth, intrauterine mortality, and high rates of spontaneous abortion are all linked to hepatitis E infection during pregnancy. Pregnant women have substantially greater mortality rates than men and non-pregnant women, especially for those who contract the disease in the third trimester, which can vary from 5% to 25%.[14,24] This may be because immunologic changes during pregnancy reduce T-cell-mediated immunity to maintain the baby in the maternal environment, making pregnant women more vulnerable to viral infections like HEV infection.[25]
HAV and HEV damage hepatocytes, causing aberrant liver functioning. The appearance of jaundice is a symptom of viral hepatitis. SGPT and SGOT are key liver enzymes that aid in protein digestion. If the liver is inflamed or harmed, they may be elevated. When there is a blockage in the liver or bile duct, ALP enzymes are increased. The present study found elevated serum bilirubin, SGOT/AST, SGPT/ALT, and ALP in HAV-positive patients, HEV-positive patients, and also in patients with coinfection which have also been reported in other studies.[20,26,27]
CONCLUSION
This study demonstrates a seasonal peak around the monsoon season and a declining trend of both HAV and HEV infection in the city probably due to improvement in public health management by the municipal authorities and increased health awareness among the people.
Ethical approval
The study approved by the Institutional Ethics Committee of Surat Municipal Institute of Medical Education and Research, number IEC/100-8/02/2022, dated 8th February 2022.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Simultaneous infection of hepatitis A and hepatitis E viruses amongst acute viral hepatitis patients: A hospital-based study from Uttarakhand. J Family Med Prim Care. 2020;9:6130-4.
- [CrossRef] [PubMed] [Google Scholar]
- Suspected spread of hepatitis A virus from a restaurant among adults in rural area of the Kerala state, India. Epidemiol Infect. 2019;147:e210.
- [CrossRef] [PubMed] [Google Scholar]
- Seroprevalence of hepatitis A and hepatitis E in patients at a teaching hospital of northern India over a period of 8 years. J Family Med Prim Care. 2022;11:567-72.
- [CrossRef] [PubMed] [Google Scholar]
- Declining trends in hepatitis A seroprevalence over the past two decades, 1998-2017, in Pune, Western India. Epidemiol Infect. 2020;148:e121.
- [CrossRef] [PubMed] [Google Scholar]
- Worldwide epidemiology of hepatitis A virus infection. J Hepatol. 1993;18(Suppl 2):S11-4.
- [CrossRef] [PubMed] [Google Scholar]
- Declining hepatitis A seroprevalence: A global review and analysis. Epidemiol Infect. 2004;132:1005-22.
- [CrossRef] [PubMed] [Google Scholar]
- Seroepidemiology of hepatitis A in voluntary blood donors from Pune, western India (2002 and 2004-2005) Epidemiol Infect. 2008;136:406-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatitis A virus: Declining seroprevalence in children and adolescents in Southeast Asia. Southeast Asian J Trop Med Public Health. 1998;29:255-62.
- [Google Scholar]
- Hepatitis E: Current status in India. Clin Liver Dis (Hoboken). 2021;18:168-72.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of hepatitis E virus viremia and antibodies among healthy blood donors in India. Indian J Gastroenterol. 2018;37:342-6.
- [CrossRef] [PubMed] [Google Scholar]
- Elimination of viral hepatitis: Evolution and India's response. Indian J Public Health. 2019;63:275-6.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of hepatitis A and hepatitis E based on laboratory surveillance data-India, 2014-2017. Am J Trop Med Hyg. 2018;99:1058-61.
- [CrossRef] [PubMed] [Google Scholar]
- Increasing burden of hepatitis A in adolescents and adults and the need for long-term protection: A review from the Indian subcontinent. Infect Dis Ther. 2019;8:483-97.
- [CrossRef] [PubMed] [Google Scholar]
- Fecal-oral transmitted hepatitis A and E prevalence in Eastern India: A 3year retrospective study. J Med Soc. 2019;33:86-90.
- [Google Scholar]
- Prevalence of enterically transmitted hepatitis viruses in patients attending a tertiary--care hospital in south India. Indian J Pathol Microbiol. 2000;43:433.
- [Google Scholar]
- Epidemiological study of hepatitis A virus and hepatitis E virus infection in patients presenting with acute viral hepatitis. Int J Curr Microbiol App Sci. 2018;7:899-904.
- [CrossRef] [Google Scholar]
- Prevalence of hepatitis A virus (HAV) and hepatitis E virus (HEV) in the patients presenting with acute viral hepatitis. Indian J Med Microbiol. 2015;33(Suppl):102-5.
- [CrossRef] [PubMed] [Google Scholar]
- Infectious hepatitis: A 3-year retrospective study at a tertiary care hospital in India. Indian J Med Microbiol. 2019;37:230-4.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of hepatitis A virus and hepatitis E virus in the patients presenting with acute viral hepatitis at a tertiary care hospital Jaipur Rajasthan. N Niger J Clin Res. 2016;5:47-50.
- [CrossRef] [Google Scholar]
- Hepatitis E virus infection during pregnancy. Virol J. 2020;17:73.
- [CrossRef] [PubMed] [Google Scholar]
- Contribution of hepatitis E virus in acute sporadic hepatitis in north western India. Indian J Med Res. 2012;136:477-82.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann Intern Med. 2007;147:28-33.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-5.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatitis E virus infection during pregnancy: Why is the disease stormy? Medicine. 2012;22:459-62.
- [Google Scholar]
- Prevalence of hepatitis A virus (HAV) and hepatitis E virus (HEV) in patients presenting with acute viral hepatitis: A 3-year retrospective study at a tertiary care Hospital in Western India. J Family Med Prim Care. 2022;11:2437-41.
- [CrossRef] [PubMed] [Google Scholar]
- A case of co infection of hepatitis A and E virus with hepatic encephalopathy. Korean J Med. 2011;80(Suppl 2):S101-5.
- [Google Scholar]






