Translate this page into:
A review on COVID-19 for medical students

*Corresponding author: Sreya Varanasi, Department of Microbiology, RAK Medical and Health Sciences University, RAK College of Medical Science, United Arab Emirates. drsvaranasi20@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Goud BK, Sharma D, Varanasi S. A review on COVID-19 for medical students. Indian J Med Sci 2021;73(1):30-5.
Abstract
It has not been very long since the SARS-CoV 2002 and MERS-CoV 2012 epidemics. Yet again, nature has introduced SARS-CoV-2, also known as COVID-19, a highly virulent strain of the coronavirus that has its origin in the city of Wuhan, Hubei Province, China. Primarily, a zoonotic infection, the virus probably found its way to humans through infected wild bats sold in the Wuhan local market. What makes the virus virulent, is its ability to infect multiple people at once through single index case. This has led to inability to contain the virus with ease posing a significant threat to national and international health-care resources and economies. The objective of this review is to highlight the key features of the novel CoV-19 infection as per existing data for better understanding of the disease.
Keywords
Novel Coronavirus
Infection
Health
Transmission
INTRODUCTION
Coronaviruses (CoVs), primarily of zoonotic origin, made their presence felt among humans through the SARS-CoV and MERS-CoV infections not so long ago. COVID-19, a new strain of the coronavirus, is currently a pandemic, with 1,780,356 reported cases and 108,828 reported fatalities worldwide.
CoVs belong to the genus Coronavirus in the Coronaviridae family of viruses. They are pleomorphic RNA viruses, 80–160 nm in size with 2–32 kb positive polarity, possessing multiple crown-shaped peplomers. Their recombination rate is very high due to continuous development of transcription errors and RNA-dependent RNA polymerase (RdRP) jumps contribute to their high mutation rate.[1,2]
There are four genera of the CoV which are α, β, γ, and δ-CoV. All four genera do not cause serious illness among humans. Variants of the α and β genera have low pathogenicity, producing mild respiratory symptoms like the common cold. The γ and δ-CoV variants tend to infect birds. SARS-CoV and MERS-CoV are β-CoVs with the potential to produce acute respiratory distress syndrome (ARDS) associated with high mortality rates.[3]
In December 2019, SARS-CoV-2 also known as the novel CoV-19 emerged in Wuhan, Hubei Province, China. Within a month, the virus made its way to other countries. On March 11, 2020, the World Health Organization (WHO) declared COVID-19 as a pandemic.[4]
EPIDEMIOLOGY
It is hypothesized that wild bats are the reservoir of SARS-CoV-2. Theory says that infected wild bats were being traded along with livestock in the Huanan Seafood Market in Wuhan from where nCoV-19 infected the index cases. Among humans, the virus is transmitted through nasopharyngeal droplets and fomites.[5-7]
On December 12, 2019, a case of pneumonia not caused by influenza or other known CoV was first reported in Wuhan. By December 31, 2019, 27 similar cases were diagnosed in the same province.[8,9] All cases reported a history of animal contact in the above-mentioned market.
On January 7, 2020, the Government of China announced that nCoV-19 was isolated from all the above cases.[10,11]
Like other coronavirus outbreaks, COVID-19 seems to exhibit high incidence in the spring season across the globe. Being the season of celebrations and gatherings, high population densities in smaller areas assist in the easy transmission of the virus among humans. Compounded by the increase in international travel, the above-mentioned factors pose significant difficulties for countries to contain the spread of the virus.[12]
GENETICS
According to research, SARS-CoV-2 is like SARS-CoV in 70% of its genome and to MERS-CoV in 50% of its genome. It has approximately 89% similarity with two bat-derived SARS-like CoVs.[13-15] Metagenomic next-generation sequencing revealed the entire 29,881 base pair genome of the virus with 6–11 open reading frames (ORFs).[16] Evolutionary analysis based on ORF1a/1b, S, and N genes suggests that SARS-CoV-2 was independently introduced from animals to humans.[17,18]
Two-thirds of the viral RNA, located in the first ORF (ORF1a/b), translates to two polyproteins, PP1a and PP1ab and encodes 16 non-structural proteins (NSP). One-third of the viral genome encodes four essential structural proteins and several accessory proteins that interfere with the host innate immune response:[19]
Spike (S) glycoprotein
Small envelope (E) protein
Matrix (M) protein
Nucleocapsid (N) protein
SARS-CoV-2 possesses the S-glycoprotein gene and receptor-binding domain (RBD) which confer the ability to be transmitted between two humans by direct contact.
When comparing SARS-CoV-2 with other known CoVs, no difference was found in the amino acid sequences of NSP7, NSP13, envelope, matrix, or accessory proteins p6 and 8b. A few differences were found in the NSP2, NSP3, spike protein, and underpinning subdomain (RBD).[20] Mutations in NSP2 and NSP3 play a role in the infectious potential and differentiation mechanism of the virus.[21]
Zhang et al.[22] found that as the virus spread across different provinces in China, its genotype exhibited several mutations.[23]
PATHOGENESIS
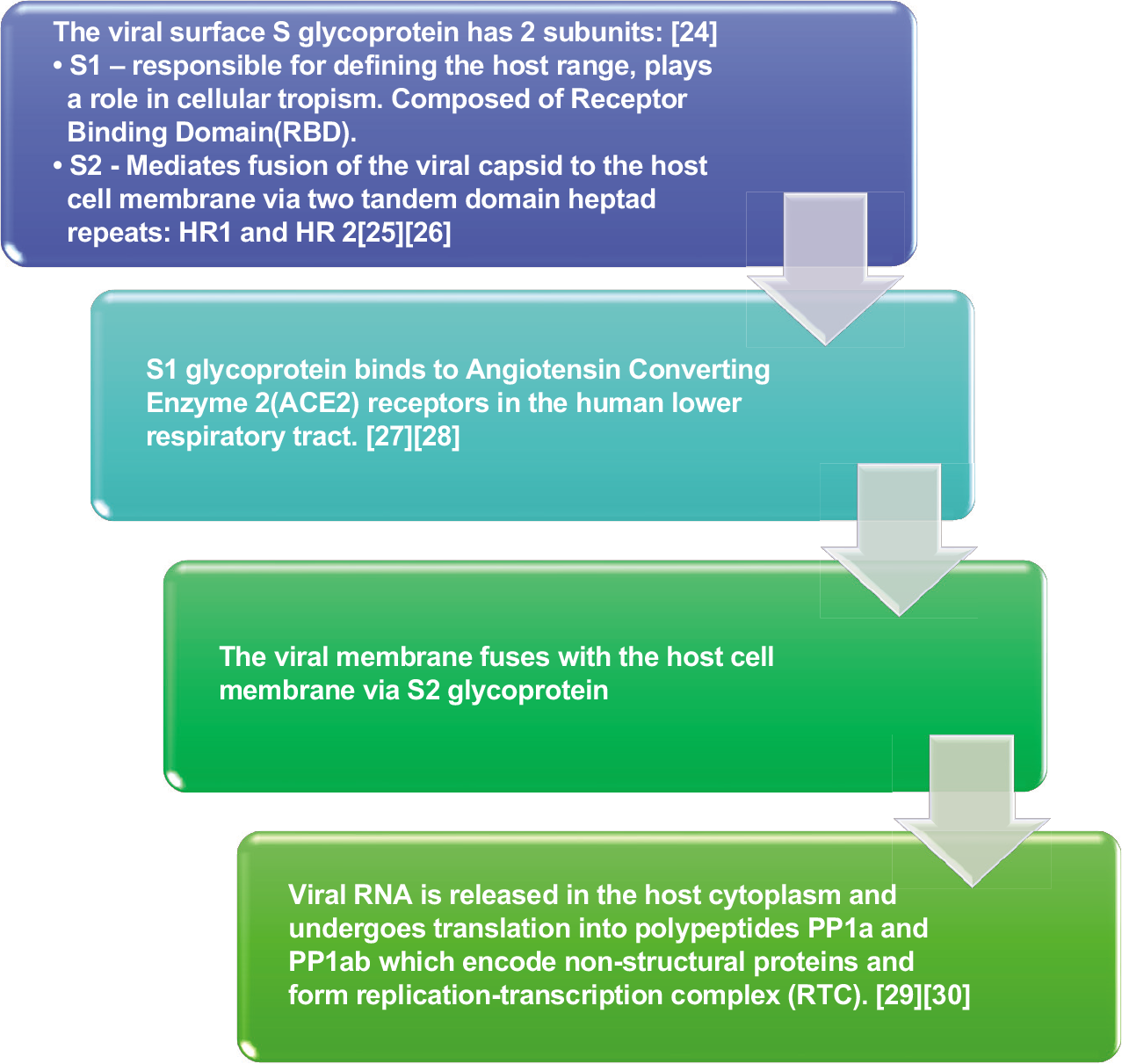
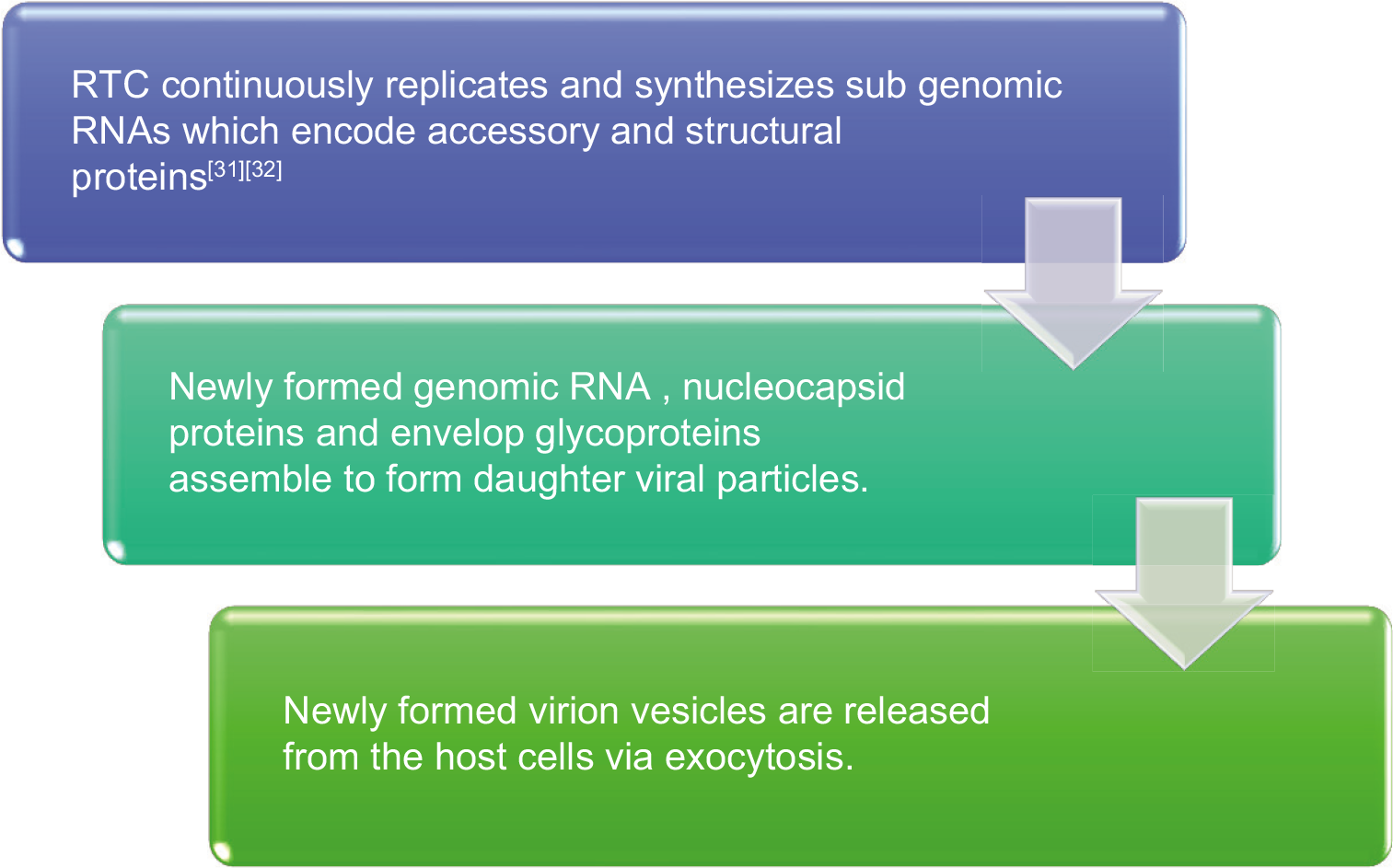
CLINICAL FEATURES
Viral load at the time of infection determines the clinical features in a host. The incubation period is approximately 14 days.
The most common clinical presentation is fever, headache, sore throat, productive wet cough, myalgia, diarrhea, and dyspnea.[33-35] The spectrum of clinical cases includes asymptomatic carriers and mild respiratory infection that recover within a week, severe progressive respiratory distress and death.[34,36]
In severe respiratory distress, respiratory system examination reveals reduced tactile fremitus, dullness to percussion, and reduced air entry associated with rales.
Majority of the case fatalities have been reported among the middle-aged and elderly population associated with multiple comorbidities such as diabetes mellitus, hypertension, coronary artery disease, liver cirrhosis, and cancer.[37]
INVESTIGATIONS
Investigations can be divided into two classes:
Tests to confirm the diagnosis of COVID 19 – a positive RT-PCR test.
Tests to monitor and assess the severity of the disease and its progression – performed in critically ill-hospitalized patients.
A patient is declared as recovered from the infection if two samples taken on different days give a negative RT-PCR result.
Routine blood tests include the following:
Complete blood count: It shows decrease in lymphocytes and white blood cells with specific reduction in CD4 and CD8 T-cells.
Arterial blood gas analysis.
Lipid profile.
Renal function tests.
Liver function tests.
Myocardial enzymes.
Urine analysis.
Inflammatory factors – interleukin 6 (IL-6), IL-10, and TNF-α.
ESR and CRP.
Coagulation profile with D-dimer
IMAGING
Chest X-rays are the initial imaging modality followed by CT thorax without contrast.
Clues to the diagnosis of COVID-19 on a chest X-ray are as follows:
Bilateral lung infiltrates along the subpleural plane and bronchial vascular bundles, singular or multiple patchy, nodular, and honeycomb- or cord-like infiltrates, particularly involving the middle and lower lobes of the lung.
Typical bilateral subsegmental ground-glass appearance
Interlobular thickening
Consolidation and thickening of bronchial walls
Atypical findings on imaging include:
Pleural effusion
Enlargement of mediastinal lymph nodes
Single or multiple solid nodules or consolidated nodules in the center of lobule surrounded by ground-glass opacities.
CT STAGING[38]
| Stages | Type of patients | Radiological features | |
|---|---|---|---|
| 1 | (No clinical symptoms) | Ultra-early stage Laboratory tests negative Positive throat swab |
Single, double, or scattered focal ground-glass opacity Nodules located in central lobule surrounded by patchy ground-glass opacities Patchy consolidation and air bronchogram the middle and lower lobes |
| 2 | Early stage (1–3 days after onset of symptoms) | Fever cough with/without sputum | Single or multiple scattered patchy or agglomerated ground-glass opacities, separated by honeycomb-like or grid-like thickened of interlobular septa |
| 3 | Rapid progression stage | (3–7 days after the onset of symptoms) | Fused and large-scale light consolidation with air bronchogram |
| 4 | Consolidation stage. | (7–14 days after onset of symptoms) | Multiple patchy consolidations with less density as compared to rapid progression stage. |
| 5 | (2–3 weeks after onset of symptoms) | Dissipation stage | Patchy consolidation or strip-like opacity evolving into grid-like thickening of interlobular septum, thickening and strip-like twist of bronchial wall and scattered patchy consolidation |
Differential diagnosis
Common cold caused by rhinoviruses
Viral pneumonia caused by influenza virus, parainfluenza virus, adenovirus, respiratory syncytial virus, and human metapneumovirus
Atypical pneumonia caused by mycoplasma and chlamydia
Bacterial pneumonia
Pulmonary involvement in vasculitis, dermatomyositis, and organizing pneumonia
Management
General guidelines include:
Supportive therapy: Rest and symptomatic treatment with antipyretics, nutritional supplements, and proton-pump inhibitors/ranitidine to prevent stress ulcers.
Self-quarantine either at home or in the hospital to break the chain of infection.
Isolation of all suspected and confirmed cases.
ICU care for critical cases: Mechanical ventilation and extracorporeal membrane oxygenation (ECMO).
VTE prophylaxis: Low-molecular-weight heparin. Contraindicated in a history of recent surgery, bleeding disorders, suspected hemorrhage, active ulcers, and allergy to heparin.
Medical therapy
Antivirals:
These drugs are not specific for treatment of SARS-CoV-2 but may help to improve the patient’s overall condition.
Example: Lopinavir, ritonavir, and interferon-alpha
Remdesivir is a 1-cyano-substituted adenosine nucleotide analog prodrug with broad-spectrum antiviral activity against several RNA viruses. The first reported case of COVID-19 in the USA was successfully treated by remdesivir.[39]
Antimalarials:
Chloroquine[40] shows great potential in the treatment of COVID-19 according to in vitro studies through the following mechanisms:
Inhibiting pH-dependent steps of viral replication[41]
Immunomodulatory effects through suppression of TNF and IL-6 production[42]
Impaired glycosylation of host cell receptors for SARSCoV-2[43-46]
Antibiotics to prevent secondary bacterial infections
Corticosteroids in rapid progressive disease: Its impact on length of hospital stay is yet to be determined.
Complications of covid-19
The following complications have been reported to date:[33,47]
ARDS
Arrhythmia
Shock – circulatory and septic
Acute kidney injury
Liver dysfunction
Prevention of COVID-19
According to the WHO, by practicing the following five steps, we can successfully prevent the spread of nCoV-19 to others and avoid being infected by it ourselves:[47]
Frequent handwashing with soap and water or an alcohol-based hand rub for at least 20 s.
Social distancing by at least 1 m or 3 ft with people who are unwell.
Covering the nose and mouth with a disposable tissue or a flexed elbow when coughing or sneezing. Dispose the used tissue immediately. Avoid touching your face repeatedly, especially if your hands are dirty.
Self-isolation (#StayHome) – especially if you feel unwell.
Seek medical attention immediately if the following symptoms develop: Fever, cough, and difficulty breathing.
At a global level, countries across the world have imposed nationwide lockdowns and public health protocols to contain the spread of COVID-19 and protect the health of their residents. Some of the measures include:
Temporary closure of international travel
Surveillance and screening of suspected individuals
Isolation of infected individuals with quarantine of their close contacts
Nationwide sterilization drives.
CONCLUSION
From the time, SARS-CoV-2 made its appearance among humankind, several researchers and health-care professionals have been risking their lives to understand the properties of the virus and aid in the recovery of infected individuals while preventing its spread among communities. By implementing lessons learned from the previous coronavirus epidemics, we can design an effective vaccine and antiviral drug that will help combat the virus. A lot more knowledge is yet to be obtained regarding the virus and the disease which will better enable us to manage patients and reduce mortality rates.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2:1804-20.
- [CrossRef] [PubMed] [Google Scholar]
- Genomic characterization of severe acute respiratory syndrome-related coronavirus in European bats and classification of coronaviruses based on partial RNA-dependent RNA polymerase gene sequences. J Virol. 2010;84:11336-49.
- [CrossRef] [PubMed] [Google Scholar]
- MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130-7.
- [CrossRef] [PubMed] [Google Scholar]
- Severe Acute Respiratory Syndrome-Related Coronavirus: The Species and its Viruses-a Statement of the Coronavirus Study Group. BioRxiv 2020:1-15.
- [CrossRef] [Google Scholar]
- The first two cases of 2019-nCoV in Italy: Where they come from? J Med Virol. 2020;92:518-21.
- [CrossRef] [PubMed] [Google Scholar]
- Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect Genet Evol. 2020;79:104212.
- [CrossRef] [PubMed] [Google Scholar]
- Estimating the Potential Total Number of Novel Coronavirus Cases in Wuhan City China. 2020. Available from: https://www.imperial.ac.uk/mrc-globalinfectiousdisease-analysis/news--wuhan-coronavirus [Last accessed on 2020 Apr 09]
- [Google Scholar]
- World Health Organization 2019-nCoV Situation Report-22. Available from: https://www.who.int/docs/defaultsource/coronaviruse/situation-reports [Last accessed on 2020 Feb 12]
- [Google Scholar]
- European Centre for Disease Prevention and Control Data: Geographical Distribution of 2019-nCov Cases. Available from: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases [Last accessed on 2010 Feb 05]
- [Google Scholar]
- Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;20:30251-8.
- [CrossRef] [Google Scholar]
- From SARS-CoV to Wuhan 2019-nCoV Outbreak: Similarity of Early Epidemic and Prediction of Future Trends. Cell Press 2020
- [CrossRef] [Google Scholar]
- A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-33.
- [CrossRef] [PubMed] [Google Scholar]
- An emerging coronavirus causing pneumonia outbreak in Wuhan, China: Calling for developing therapeutic and prophylactic strategies. Emerg Microbes Infect. 2020;9:275-7.
- [CrossRef] [PubMed] [Google Scholar]
- From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11:59.
- [CrossRef] [PubMed] [Google Scholar]
- RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg Microbes Infect. 2020;9:313-9.
- [CrossRef] [PubMed] [Google Scholar]
- A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-3.
- [CrossRef] [PubMed] [Google Scholar]
- Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181-92.
- [CrossRef] [PubMed] [Google Scholar]
- Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325-8.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-2019: The role of the nsp2 and nsp3 in its pathogenesis. J Med Virol. 2020;92:1-4.
- [CrossRef] [PubMed] [Google Scholar]
- Origin and evolution of the 2019 novel coronavirus. Clin Infect Dis 2020:ciaa112.
- [CrossRef] [PubMed] [Google Scholar]
- Poultry farms as a source of avian influenza a (H7N9) virus reassortment and human infection. Sci Rep. 2015;5:7630.
- [CrossRef] [PubMed] [Google Scholar]
- Current advancements and potential strategies in the development of MERS-CoV vaccines. Expert Rev Vaccines. 2014;13:761-74.
- [CrossRef] [PubMed] [Google Scholar]
- Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell Mol Immunol 2020
- [CrossRef] [PubMed] [Google Scholar]
- Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microbes Infect. 2020;22:74-9.
- [CrossRef] [PubMed] [Google Scholar]
- ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79:14614-21.
- [CrossRef] [PubMed] [Google Scholar]
- Structural insights into coronavirus entry. Adv Virus Res. 2019;105:93-116.
- [CrossRef] [PubMed] [Google Scholar]
- Host factors in coronavirus replication. Curr Top Microbiol Immunol. 2018;419:1-42.
- [CrossRef] [PubMed] [Google Scholar]
- Coronavirus transcription: A perspective. Curr Top Microbiol Immunol. 2005;287:31-55.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of novel subgenomic RNAs and noncanonical transcription initiation signals of severe acute respiratory syndrome coronavirus. J Virol. 2005;79:5288-95.
- [CrossRef] [PubMed] [Google Scholar]
- The C-terminal domain of the MERS coronavirus M protein contains a trans-Golgi network localization signal. J Biol Chem. 2019;294:14406-21.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020:NEJMoa2002032.
- [CrossRef] [Google Scholar]
- Novel Coronavirus, Wuhan, China. 2020. Available from: https://www.cdc.gov/coronavirus/2019-nCoV/summary.html [Last accessed on 2020 Apr 08]
- [Google Scholar]
- Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
- [CrossRef] [Google Scholar]
- Beijing union medical college hospital on “pneumonia of novel coronavirus infection” diagnosis and treatment proposal (V20) Med J Peking Union Med Coll Hosp. 2020;9:29.
- [CrossRef] [PubMed] [Google Scholar]
- A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia. Military Med Res. 2020;7:1-23.
- [Google Scholar]
- First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-36.
- [CrossRef] [PubMed] [Google Scholar]
- Chloroquine analogs as antimalarial candidates with potent in vitro and in vivo activity. Int J Parasitol Drugs Drug Resist. 2018;8:459-64.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of chloroquine on viral infections: An old drug against today's diseases? Lancet Infect Dis. 2003;3:722-7.
- [CrossRef] [Google Scholar]
- Quinoline-based antimalarial drugs: A novel class of autophagy inhibitors. Neurosurg Focus. 2015;38:E12.
- [CrossRef] [PubMed] [Google Scholar]
- Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19:1313-7.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212:1904-13.
- [CrossRef] [PubMed] [Google Scholar]
- Ribavirin and interferon alfa-2a for severe middle east respiratory syndrome coronavirus infection: A retrospective cohort study. Lancet Infect Dis. 2014;14:1090-5.
- [CrossRef] [Google Scholar]
- Clinical analysis of 190 cases of outbreak with atypical pneumonia in Guangzhou in spring, 2003. Zhonghua Yi Xue Za Zhi. 2003;83:713-8.
- [Google Scholar]
- Use of glucocorticoid in treatment of severe acute respiratory syndrome cases. Zhonghua Yu Fang Yi Xue Za Zhi. 2003;37:233-5.
- [Google Scholar]
- Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-9.
- [CrossRef] [PubMed] [Google Scholar]
- Advice for Public. 2020. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public [Last accessed on 2020 Apr 09]
- [Google Scholar]






