Translate this page into:
An epidemiological review of pancreatic cancer with special reference to India

*Corresponding author: Rajshree H. Gaidhani, Department of Medical Records, Biostatistics and Epidemiology, Tata Memorial Centre, Centre for Cancer Epidemiology, ACTREC Campus, Mumbai, Maharashtra, India. drrajshreegaidhani@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gaidhani RH, Balasubramaniam G. An epidemiological review of pancreatic cancer with special reference to India. Indian J Med Sci 2021;73(1):99-109.
Abstract
Pancreatic cancer (PC) is a rare cancer site, ranked 14th in incidence and 7th in mortality in the world. In India, pancreas ranks 24th with 10860 new cases (1.03%) and 18th in mortality. Although PC is a rare site, it is a leading mortality site across the globe and very little data are available about the epidemiology of PC. It is hypothesized that due to the changing lifestyle globally and, in India, the rates of PC will increase in the near future. Thus, this study aims to report PC incidence, mortality, globally but with more emphasis with respect to Indian population and associated factors for PC, since changing lifestyle has had an impact on the occurrence of the disease over the years. Incidence and mortality rates are obtained from GLOBOCAN-2018 and cancer incidence in five continents (CI5- XI), and Indian Council of Medical Research publication on Indian cancer registry database. Incidence is higher in elderly population (more than 50% in 65–75 years). The incidence is highest among Northeastern Indian regions. Risk factors include smoking, high alcohol consumption, non-vegetarian diets (modifiable) and age, race, and the genetic predispositions (non-modifiable) risk factors. No case–control studies on risk factors are reported yet based on the Indian population. Increase of PC numbers is a cause for concern, since it mostly manifests on the lifestyle factors, which is rapidly changing in India, like in other parts of the globe. This study will be useful in giving some leads on the PC’s possible causes and thereby help in formulating strategies for reducing the burden of this disease.
Keywords
Risk-factors
India
Incidence-rates
Pancreatic-cancer
Epidemiology-review
INTRODUCTION
As per the GLOBOCAN 2018 estimates, pancreatic cancer (PC) ranks 14th in the world,[1] and the incidence rates varies across the globe.[2] India is no exception to this phenomenon and variations have been observed in different regions, northeast showing higher rates.[3,4]
The objective of the study is to undertake a review on the PC and elucidate the incidence, mortality, and risk factors, globally but with special emphasis with respect to Indian population. The study has a premise and hypothesized that due to the changing lifestyle globally and in India, the rates of PC will increase in the near future. Dietary habits, tobacco use, smoke and smokeless, and not sufficient physical activity will enhance the risk besides other associated factors. To this effect, newer strategies need to be worked out by health professionals to advice the government on these issues and provide guidelines for authorities to reduce the burden of this disease in the years to come.
MATERIAL AND METHODS
The study being a review article, the primary source of information is from the published reports IARC,[1] GLOBOCAN-2018,[1] and CI5-XI[2] and Indian Council of Medical Research publication as NCDIR (2016)[3] and NCRP (2013).[4] Risk factors were obtained from various scientific publications reported in PUBMED, SCIENCEDIRECT databases. Reliable survival statistics are referred and presented.[5]
RESULTS
As per the estimates on cancer reported by GLOBOCAN (2018), there would be 18.1 million new cases and 9.6 million deaths [Table 1].[1]
| All cancers | |
|---|---|
| Number of new cases | 18.1 million |
| Number of deaths | 9.6 million |
| Pancreatic cancer | |
| Number of new cases | 458,918 (14thrank) |
| Number of deaths | 432,242 ( 7thrank) |
| Incidence rates (ASR) - both sexes | 4.8 |
| Males | 5.5 |
| Females | 4.0 |
| Mortality rates (ASR) - both sexes | 4.4 |
| Regional variation | Incidence rates (ASR) per 10 |
| Males | |
| Highest (top 3) | |
| Eastern Europe | 9.9 |
| Western Europe | 9.5 |
| North America | 8.7 |
| Lowest | |
| South Central Asia | 1.1 |
| Females | |
| Highest (top 3) | |
| Western Europe | 7.2 |
| Northern America | 6.5 |
| Northern Europe | 6.4 |
| Lowest | |
| South Central Asia | 1.0 |
| HDI** | |
| Very high HDI | 7.2 |
| High HDI | 1.2 |
| Low HDI | 1.2 |
PC
Incidence and mortality - global burden
Globally, based on GLOBOCAN (2018) estimates, 0.46 million new PC cases and 0.43 million deaths due to PC are reported annually [Table 1] and the incidence and mortality rates are 4.8 and 4.0, respectively. It is seen that the rates in high human development index (HDI) countries are three-fold compared to low HDI countries.[1]
Regional variations, as shown in Table 1, indicate that incidence rates of PC in males were highest in Eastern Europe (9.9), followed by Western Europe (9.5), North America (8.7), and lowest in South Central Asia (1.1); among females, the rates were 7.2 in Western Europe, followed by Northern America (6.5), Northern Europe (6.4), and lowest in South Central Asia (1.0);[1] In the future, it is projected that the deaths due to PC will be ranked third, breast cancer being ranked third as of now.[6]
It is seen in Table 2 that in male population, incidence rates were higher in Republic of Moldova (15.3) and Hungary (12.9), while in female population, Hungary (9.1), and UAE (10.1) showed higher rates; the mortality rates among males in Republic of Moldova (12.3) and Hungary (11.2) showed the higher rates while in females, rates in UAE (10.0) and Hungary (7.9) were higher.[1] In Table 2, it is observed that in all the countries, the incidence rates were higher than mortality rates in both the sexes, except in Russian Federation; it is observed that in Russian Federation, mortality rates were higher in both males and females than its incidence.[1]
| Age-standardized incidence rates (ASR) per 105 | Age-standardized mortality rates (ASR) per 105 | ||||
|---|---|---|---|---|---|
| Country | Female | Male | Country | Female | Male |
| India | 0.88 | 0.81 | India | 0.84 | 0.79 |
| United Arab Emirates | 10.1 | 2.0 | United Arab Emirates | 10.0 | 2.0 |
| World | 4.0 | 5.5 | World | 3.8 | 5.1 |
| China | 4.2 | 6.2 | China | 4.2 | 5.6 |
| Canada | 5.9 | 7.0 | Canada | 5.1 | 6.7 |
| Australia | 6.4 | 7.5 | Australia | 4.8 | 7.1 |
| United Kingdom | 6.3 | 7.9 | United Kingdom | 5.3 | 6.7 |
| Italy | 6.5 | 8.5 | Italy | 5.7 | 7.7 |
| United States of America | 6.6 | 9.0 | United States of America | 5.6 | 7.7 |
| Sweden | 6.9 | 8.6 | Sweden | 6.9 | 8.6 |
| Germany | 7.4 | 9.3 | Germany | 6.6 | 9.2 |
| Russian Federation | 5.5 | 9.6 | Russian Federation | 5.6 | 10.0 |
| France | 7.1 | 10.8 | France | 6.1 | 9.5 |
| Japan | 7.7 | 11.7 | Japan | 6.2 | 9.5 |
| Hungary | 9.1 | 12.9 | Hungary | 7.9 | 11.2 |
| Republic of Moldova | 6.7 | 15.3 | Republic of Moldova | 5.5 | 12.3 |
Differences in incidence rates between registries across the world
As per the CI5-XI,[2] there are gender-specific variation in PC [Table 3]. The incidence rates differed among men; the incidence rates were higher in Italy- Sondrio (12.0), Czech Republic (11.8), and Slovakia (11.8), while it was lower in India-Dindigul (0.5), India-Tripura (0.6), and South Africa- Eastern Cape (0.7).[2] Similarly among women, rates were higher in Italy-Sondrio (9.0), Czech Republic (8.0), Germany-Bremen (7.8)[2] and lower in India-Tripura (0.3), India-Dindigul (0.3), and South Africa-Eastern Cape (0.4).[2]
| Males | Rates | Females | Rates |
|---|---|---|---|
| Country, Registry | ASR | Country, Registry | ASR |
| Italy, Sondrio | 12.0 | Italy, Sondrio | 9.0 |
| Czech Republic | 11.8 | Czech Republic | 8.0 |
| Slovakia | 11.8 | Germany, Bremen | 7.8 |
| Japan, Niigata | 11.7 | Slovakia | 7.6 |
| France, Herault | 11.5 | Austria, Tyrol | 7.6 |
| China, Hengdong | 1.8 | China, Hengdong | 1.1 |
| Thailand, Khon Kaen | 1.0 | Thailand, Khon Kaen | 0.8 |
| India, Sikkim State | 0.8 | India, Sikkim State | 0.8 |
| South Africa, Eastern Cape | 0.7 | South Africa, Eastern Cape | 0.4 |
| India, Tripura | 0.6 | India, Tripura | 0.3 |
| India, Dindigul, Ambilikkai | 0.5 | India, Dindigul, Ambilikkai | 0.3 |
It is seen from Table 4, that the proportion of PC cases in Bhutan and China is more than two-fold than that seen in India, which is similar to that observed in the north-eastern regions of India.[2]
| Country | Incidence | Mortality | ||
|---|---|---|---|---|
| Number of cases | Rank (% of all cancers) | Number of cases | Rank (% of all cancers) | |
| Bangladesh | 1400 | 21 (1.07) | 1373 | 18 (1.44) |
| Bhutan | 18 | 10 (3.40) | 18 | 8 (3.98) |
| China | 116291 | 9 (2.83) | 110390 | 6 (4.02) |
| India | 10860 | 25 (1.01) | 4752 | 28 (0.66) |
| Nepal | 497 | 16 (2.16) | 455 | 13 (2.64) |
| Pakistan | 1070 | 25 (0.72) | 1008 | 21 (0.99) |
| Sri Lanka | 172 | 24 (0.80) | 155 | 21 (1.21) |
| Vietnam | 965 | 22 (0.63) | 899 | 18 (0.83) |
Incidence and mortality in India
The rates are obtained from the Population-Based Cancer Registries (PBCR) in India. It is projected that the number of cases will escalate from 10969 in 2015 to 11655 in year 2020 though it is not included among the ten leading cancer sites in India.[3] Mizoram has the highest age-adjusted incidence rates and northeastern region has significantly high number of PC cases as compared to any other regions across the country.
In Figure 1, it is seen that, among males, Mizoram (3 per 105) showed highest rates followed by Mumbai (2.8) and Thiruvananthapuram (2.6), whereas in females, Mumbai (2.2 per 105) showed highest rates followed by Delhi (1.7) and Thiruvananthapuram (1.5).[3] Although Mumbai and Mizoram share similar incidence rate for males, it is not so in females.

- Age-adjusted incidence rates (per 105) of PC in Indian Population-Based Cancer Registries (2012-2014).
Time trends across the globe and in India
Global trends
Cancer trends are an important indicator of the changing pattern of occurrence of cancer and provide insight into the possible reasons/factors that may have contributed to the phenomenon. The age standardized incidence rate data from year 1988 to 2012 for age (0–85+ years) showed some interesting trends in both the sexes, as reported in CI5-Vol XI.[2] The incidence rates in males in USA, as shown in Figure 2, though with fluctuations did not show much change, over the period and China as well did not show any significant change in rates; however, France, Bas-Rhin had shown 30% increase, a 20% increase over the period in Japan, Miyagi, and two-fold increase in India, Chennai, and a 50% increase in rates in Italy, Parma.[2]
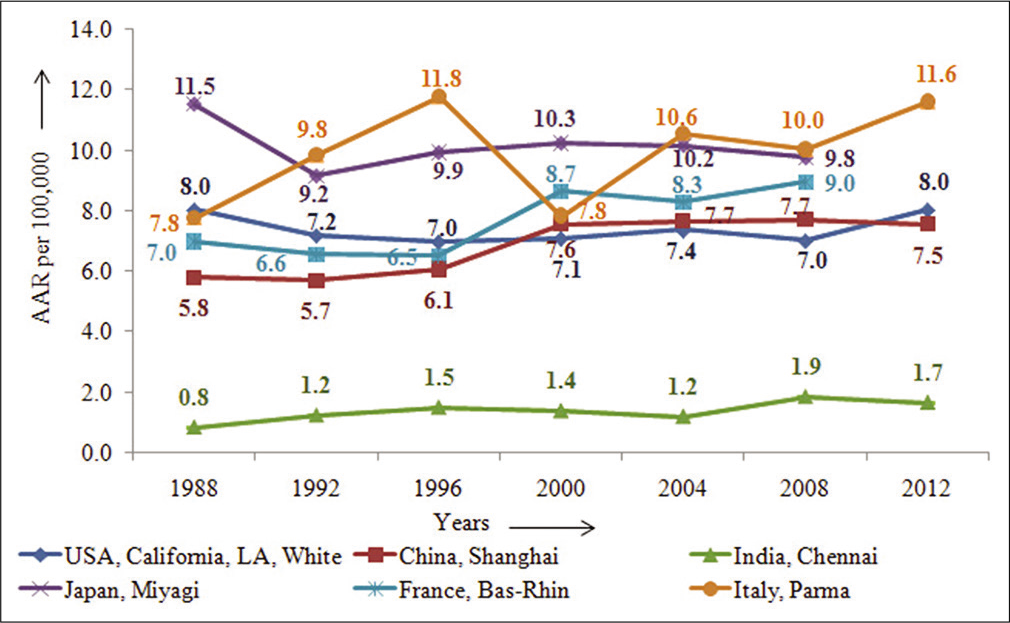
- Time-trends of pancreatic cancer in some selected countries across the globe –males.
Similarly, among the females, as seen in Figure 3, China, Shanghai, France-Bas-rhin, and Italy, Parma showed about 50% increase over the period from 1988 to 2012, while registries as USA, India, and Japan did not show any appreciable change in incidence rates.[2]
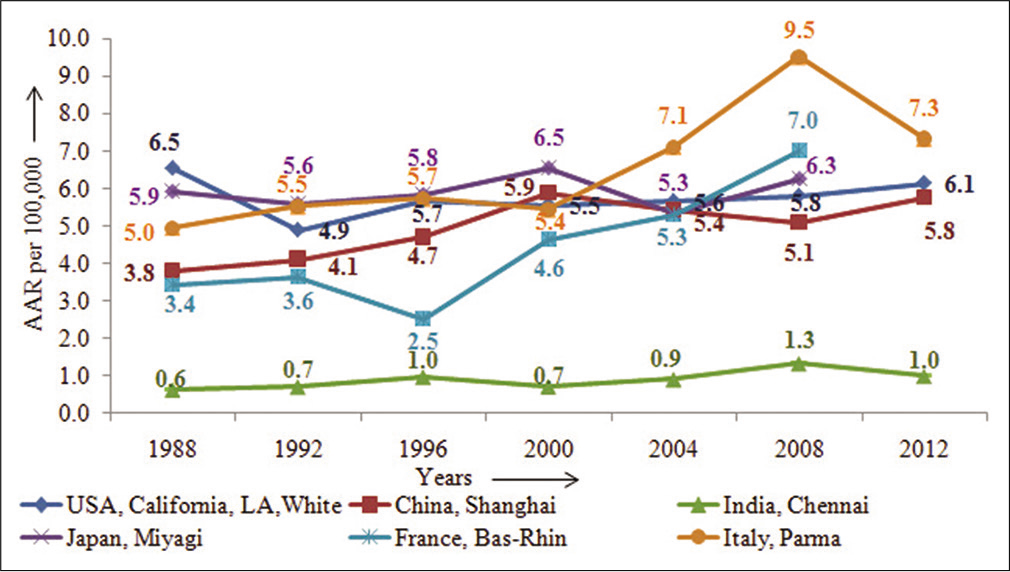
- Time-trends of pancreatic cancer in some selected countries across the globe – females.
Time trends in Indian PBCR
The comparison between four PBCR, Bengaluru, Chennai, Delhi, and Mumbai, as displayed in Figure 4, is based on data from a network of Indian cancer registries;[4] it shows that the rates have increased more than two-fold. Mumbai had shown a steady increase in the AAR from 2.1 in 1982 to 2.8 in 2012, Bengaluru AAR has tripled from 0.6 in 1982 to 1.8 in 2012, Delhi showing increasing trends from 1990 to 2002 which after a decline, again peaked to 2.5 in 2012, and in Chennai it peaked in 1997 (2.0) followed by a decline in 2002 and eventually increased to 1.8 in 2012; this trend as seen in Indian registries indicated an inconsistent trend in the rates of PC over the years.[3,4]
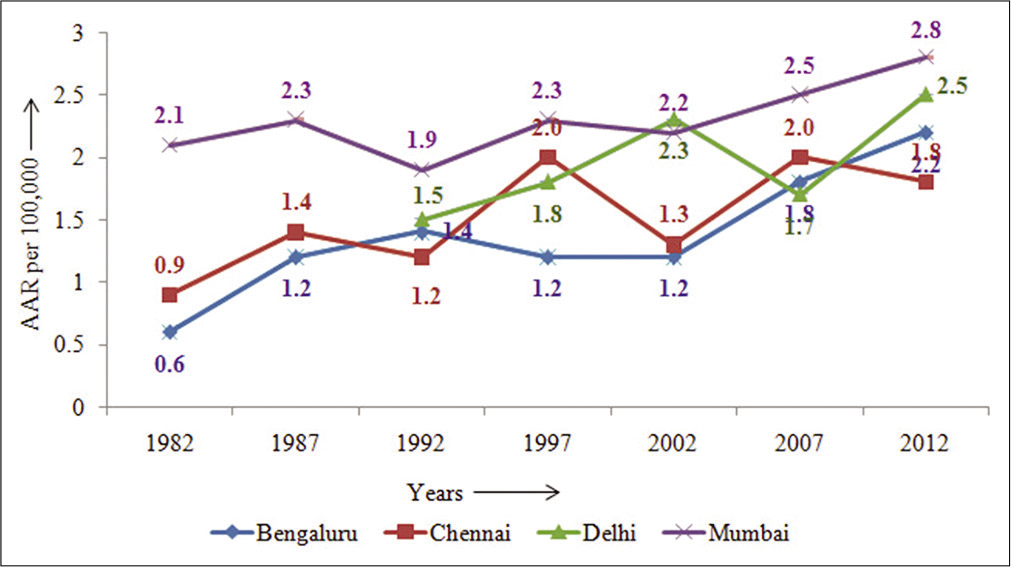
- Trends in Indian population-Based Cancer registries: Age-adjusted incidence rates (per 105) (1982–2012)– males.
Hospital-based cancer registries (HBCR)
HBCR are important source for population registries, where a major proportion of cases is diagnosed. India has a large network of HBCRs. The frequency of PC cases seen in males (very few female cases) in different hospital registries across India, as Mumbai, Chennai, Bengaluru, Delhi, Mizoram, Thiruvananthapuram, and Silchar are shown in Figure 5 reveals that the highest number of cancer cases is seen in Thiruvananthapuram and Chandigarh in both the males and females.[7]
![Number of pancreatic cancer cases in HBCR across India (NCDIR, 2016).[7]](/content/101/2021/73/1/img/IJMS-73-099-g005.png)
- Number of pancreatic cancer cases in HBCR across India (NCDIR, 2016).[7]
Trend of PC seen in the Tata Memorial Hospital (TMH)
TMH is a premier cancer hospital in India setup seven decades ago. Figure 6 showing 5-yearly interval data of PC cases which indicates an increasing trend in the proportion of PC cases in both the sexes over the years.[8-14]

- Distribution of pancreatic cancers seen over the years in Tata Memorial Hospital: 1987–2017.
In TMH, it is seen from Figure 6 that there has been a sevenfold increase in the cases over the years from 1987 to 2012 in both sexes; however, from 2012 to 2017, there has been a two-fold increase in both sexes. This could probably be due to awareness of the cancer facility expertise in diagnosis and treatment in TMH that has shown the increase in registrations.
HDI
HDI has profound effect on the inequalities with respect to PC. Higher the HDI score, higher is the rates [Figure 7]. The incidence rates in High-HDI (4.6) are three-fold or six-fold in very high-HDI (7.2), when compared to low-HDI (1.2) countries; similarly, the mortality rates are also either threefold or six-fold in high-HDI or very high-HDI countries as compared to low-HDI countries [Figure 7].[15]
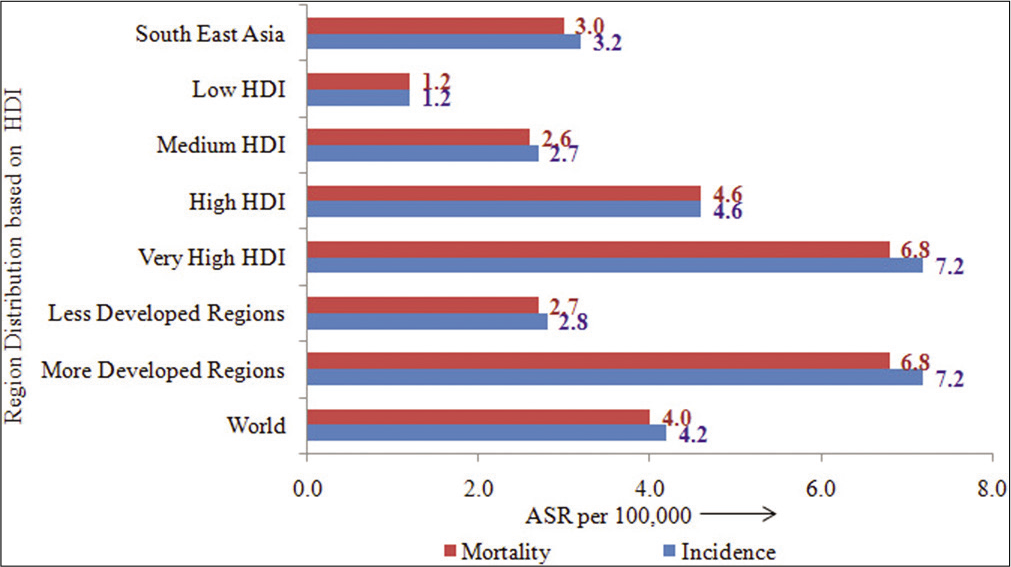
- Age-standardized rates (per 105) of pancreatic cancer by human development index.
Ethnic variations and migration impact
Incidence rates according to racial origin and migrant population are very well elucidated in Cancer Incidence in Five Continents Vol XI.[2] The differences in incidence rates by racial origin and migration are shown in Figure 8.
It is observed that the incidence rate for PC, in general, is higher in American–Blacks (11.8) as compared to American–Whites (8.6) population. As per data from year 1973–2013 (SEER-9 registries), higher incidence rate is observed among American–Black males (11.2), American– Black females (9.4) than that seen in American–White males (9.1) and American–White females (6.4), indicating that there is a ethnic variations in PC occurrence in the population between the races.[2]
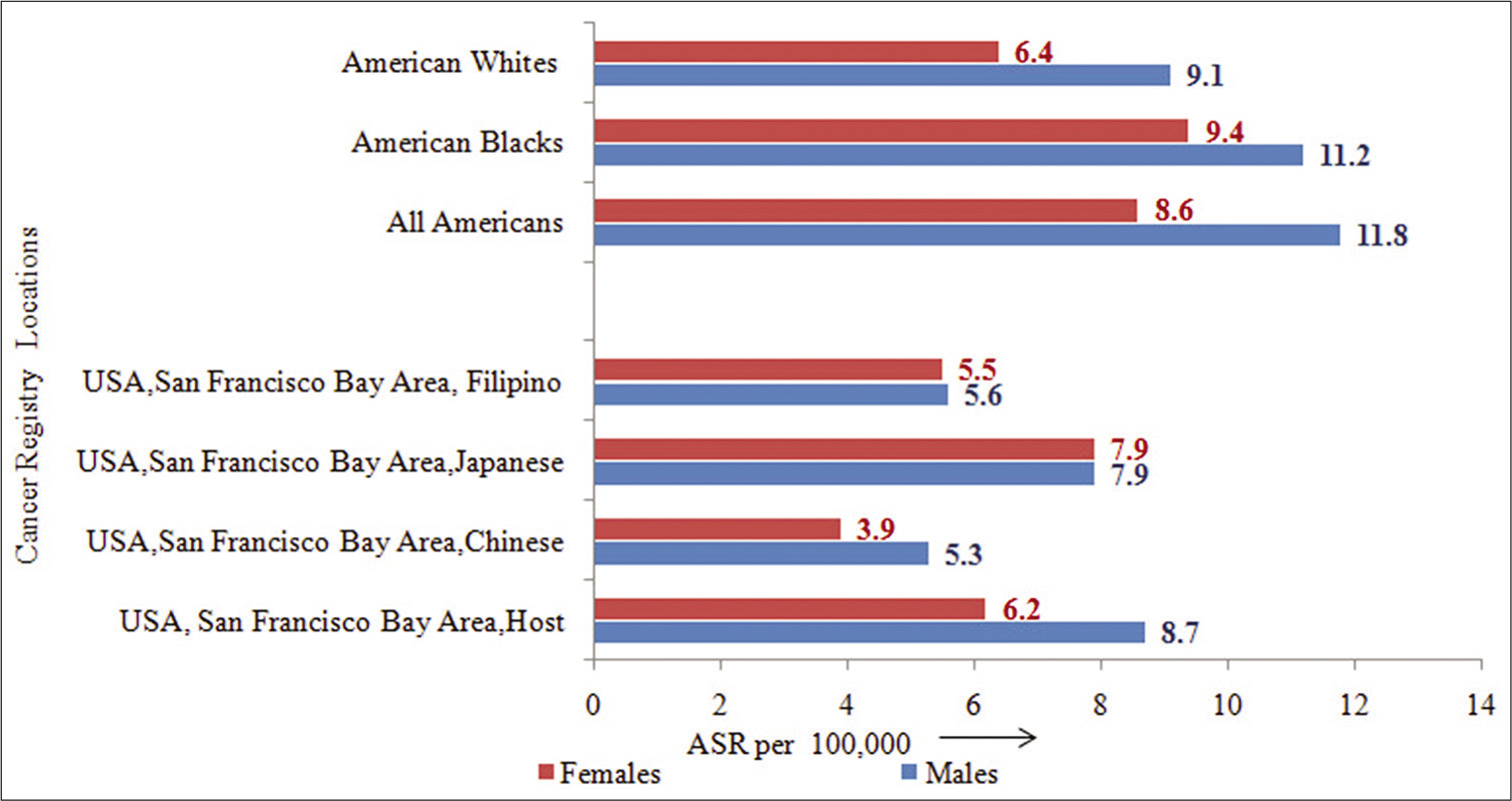
- Incidence rates (ASR) by racial origin and migration status.
Similarly insight into the migration effect is shown in Figure 8. It reveals that among males, the rates observed in USA, San Francisco Bay Areas of Chinese (5.3), Japanese (7.9), and Filipino (5.6) are different than those observed in the host USA, San Francisco Bay Area (8.7) population. Similarly among females, the rates observed in USA, San Francisco Bay Area of Chinese (3.9), Japanese (7.9), and Filipino (5.5) are different than those observed in the host USA, San Francisco Bay Area (6.2) population. In both the sexes, it indicated clearly that migration has no significant effect on PC occurrence in the population, over the years.[2]
The large variation in time for migrants to adopt the host population’s cancer risk suggests that risk factors have organ-specific effects, or operate at different times in life. The persistent difference in cancer incidence several generations after migration supports the idea that living in the host country is not, alone, sufficient to modify cancer risk for all cancer sites to the level of the host population. Although the migration effect, if any, can be partially explained by known etiologic factors, a large proportion of the changing risk remains.
Diagnostic evaluation and treatment modalities
Apart from the routine investigations, the tumor marker serum CA 19-9 is measured for PC (sensitivity 80%, and specificity 90%);[16] however, this cannot be considered as confirming investigation for PC as the marker is elevated in several other non-malignant conditions of liver, pancreas, and gastric cancer.[17] Imaging techniques help in accurate pre-operative staging of PC. This helps to avoid surgical explorations and to classify the disease in resectable, borderline resectable, locally advanced, and un-resectable metastatic stage of disease. For this MRI, multidetector computed tomography and magnetic resonance cholangiopancreatography show comparable sensitivity and specificity.[10,18] It is important to mention the importance of triphasic/pancreatic protocol CT scan for accurate assessment of lesion and involvement/relation with surrounding vasculature, thus classifying tumors as resectable/borderline resectable and locally advanced unresectable as per the NCCN criteria.[19]
For resectable and borderline-resectable PC, radical surgical resection is the best option for treatment with adjuvant chemotherapy (fluorouracil, leucovorin, or gemcitabine) that improves the overall survival. It is suggested that the patients higher than T2-stage and node-positive resections should be offered adjuvant chemoradiation;[20] also, neoadjuvant chemotherapy with or without radiotherapy is an evolving and preferred approach for BRPC.
As a case in point, between 2007 and 2012, it was observed that 95% of cases were diagnosed at non-localized stage of disease (included direct extent, regional lymph node involvement, too advanced, and metastasis). Cases treated outside and recurrence were excluded. There was a stage shift in 2012 approximately by 5%.[12-14]
Cancer survival
It is known that PC is an aggressive disease and has limited options for cure. The 5-year survival rate for PC was reported to be <5% in earlier studies.[21] In USA, European countries, Japan, and China, PC is reported as one of the most common type of cancers and the mortality/incidence ratio was 98%.[15] The 5-year survival rate was 6% (ranged from 2% to 9%) and there was little difference in the survival rates between the developed and developing countries.[21,22]
Based on the USNCI data for PC, all racial origins and genders, 9.4% diagnosed in localized-stage of disease and 52% in distant-metastasis stage showed a 5-year survival rates of 29.3% and 2.6%, respectively.[23] Some inter-country survival differences for PCs existed in Europe, where the 5-year survival rate was observed to be less than 3%, in both sexes in England and Wales;[24] it was 3.8% in Denmark and Sweden[25] and 1.2% in Italy.[26]
Survival analysis of PC patients diagnosed between 1990 and 1994 in 22 European countries was reported by EUROCARE. Highest 5-year survival rates were observed in Estonia (7.0%) and in Czech Republic (7.5%), among men and women, respectively, and lowest survival rates were observed in Malta (0%) and Slovenia (1.3%) in men in women, respectively;[21] however, in the early 21st century, survival rates in Germany were reported to be 9.0%.[27]
As per the latest survival estimates reported by CAN SURVII (report published by IARC),[28] the 5-year relative survival rates in Chennai (1990–1999) were 8.0% while it was 15.2% in Mumbai (1992–1999). It was reported as 9.5 % in Shanghai (1992–1995) and 31.0% in Tianjin (1991–1999), thus indicating there are gaps and biases in the data of this observation.
Etiology and risk factors
Identification of risk factors and methods to either reduce or eliminate the factors, play an important role in prevention of cancer. The risk factors may be classified on the basis of lifestyle, modifiable factors, and factors on which an external intervention/influence cannot modify the risk and termed as not-modifiable risk factors.
For PC modifiable risk factors include tobacco smoking, excessive alcohol consumption, life-style disorders such as obesity, dietary habits, and exposure to hazardous chemicals. Non-modifiable risk factors attributing to PC include age, gender, race, and inherited genetic syndromes, conditions such as chronic pancreatitis, geographical conditions such as antipodal locations.
Modifiable risk factors
Tobacco consumption
Smoking of tobacco is known to be one of the causative risk factor for PC. Cigarette smoking is the only established risk factor;[29] those who smoked had a two-fold enhanced risk compared to those who never smoked, and about 20–30% of PCs were due to cigarette smoking.
Smoking is higher in males as compared to females and a major risk factor attributing to cancer cases in males.[30] It is interesting to know that the risk of developing PC reduces after cessation of smoking; it is been projected that 25% of deaths due to PC in United States can be prevented with smoking cessation.[31]
Alcohol consumption
In a case–control study of PC, it was reported that heavy alcohol drinkers had greater risk than non-drinkers, and risk among men was higher (OR = 2.0).[32] In a similar study conducted elsewhere, concluded that those with high alcohol intake had elevated risk, while those with low-to-moderate alcohol intake had little or no effect on the risk of PC.[33]
Diet
High consumption of grilled and processed meat, fried food, food rich in cholesterol, and nitrosamines is directly associated with increased risk of developing PC by 30–50%.[34,35] A qualitative analysis of case–control studies revealed that high intake of vegetable and fruits was associated with decreasing the risk for the development of PC.[36] However, there are inconsistent findings on red meat as a risk factor.[37,38]
Obesity and physical inactivity
One of the earlier meta-analysis study reported a positive correlation between obesity, and increased BMI as a risk factor for PC in both sexes.[39] In another meta-analysis study consisting of 21 independent prospective studies concluded that the RR for BMI was 1.16 in men and 1.10 in women.[40] The strongest association observed was between BMI and PC mortality in racial disparity study arising due to smoking/ obesity in Black men.[41]
It was reported that obese people were 20% more likely to develop PC and lack of physical activity might increase PC risk further.[5]
Workplace exposure to certain chemicals
The American Cancer Society reported that heavy exposure at work to certain chemicals used in the dry cleaning and metal working industries might increase the risk of PC.[5]
Not-modifiable risk factors
Age at diagnosis
It is known that risk for cancer increases with advancing age and PC is no exception.
Occurrence of PC is extremely rare in those below the age of 30 years, and is common in those above the age of 55 years, majority being in their seventh and eighth decades of life.[42,43]
The age interval at which the incidence is highest differs between countries. In India, the incidence peaks in their sixth decade of life whereas in the United States, it is in the seventh decade of life.[42]
Gender
The incidence of PC in men is higher than women and it is more pronounced while comparing highly developed and less developed countries, and it is seen in Indian population as well.[15]
Studies on evaluating gender-specific hormonal risk factors causal role in susceptibility to the PC are reported in the literature;[44,45] however, one of the studies negated association of reproductive factors in women.[44] The study concluded that the findings indicated that there were other factors as environmental conditions or habits like smoking which till recent past were more common in males than females.[42]
Race
Black-origin have been diagnosed with PC at 48% and died at a 37% higher rate than White-origin;[46] it was concluded that there could be some other lifestyle risk factors that contributed to this outcome.
Family history
It has been estimated that the risk of developing PC in an individual with two affected family members to be 6.4-fold and 32-fold for three or more affected family members.[47] The sporadic cases of PC in family do not appear to be a potential risk factor in the development of PC.[48] Except for BRAC2, so far, no gene has been identified in majority of familial PC cases.[49]
Inherited genetic syndromes
As per the report by the American Cancer Society, some of the inherited gene (mutations) can be transmitted from parent to child who might cause 10% of PCs. Sometimes these changes result in syndromes that include increased risks of other cancers/other health problems.[5]
Diabetes mellitus
Diabetes has direct association with the risk of development of PC. Both, Type-1 and Type-2 diabetes has risk of developing PC.[5] Study on Italian population had shown that diabetes is believed to be cause for approximately 10% of PC.[50] Several other studies in various populations had also provided evidence that PC and diabetes are strongly linked with respect to onset of PC.[51-54]
Elaborate study of association between diabetes and PC is very much required and will help to use condition of diabetes as a marker of disease and to identify the population at risk for screening.[55]
Chronic pancreatitis
As per the American Cancer Society reports, chronic pancreatitis, a long-term inflammation of the pancreas, though increased risk for smokers, may never develop PC; they attribute this risk to an inherited gene mutation.[5]
DISCUSSION
PC is a rare cancer and is ranked 4th in incidence and 7th in mortality in the world.[1] By 2030, it may become the second leading site in mortality. The incidence rates are highest among the European countries in males and females and the lowest incidence rates were observed in Southeast Asian countries.[2] Gender-wise, the rates are higher in males than compared to females, showing male predominance.[1] The incidence rates are higher in American–Black than compared to American-White population.[2] HDI has a deep impact on the incidence and mortality rates; higher the HDI, higher are the incidence and mortality rates.[1] The ASR with respect to incidence and mortality for Southeast Asia are similar to countries classified between high and medium HDI. Among the Southeast Asian countries, China, is leading in the percentage of incidence and mortality followed by Bhutan, Nepal, and India.[1]
In India, PC is not among the ten leading cancer sites and is ranked 21st in males and 17th in females; in males Mizoram has the highest AAR, followed by Mumbai, Thiruvananthapuram, and Delhi while in females, Mumbai showed highest rates followed by Delhi, Bengaluru, and Thiruvananthapuram. Inconsistent trend is seen in Indian registries that could possibly be related to lack of reporting of all cases in registries.[3,4] In India, among the various HBCRs, Mumbai recorded the highest number of cases, followed by Thiruvananthapuram and Chandigarh.[7] TMH is the premier cancer hospital in India. Over the period of 1987– 2017, TMH showed an increasing trend in both the sexes.[8-14] Over a period of three decades, there has been a three-fold increase in number of cases; most of the cases are diagnosed in advanced stage of disease.[10-14] Between 2007 and 2012, since 95% of cases were diagnosed at non-localized stage of disease which is in contrast to world literature where 15– 20% present are in operable stage, the authors would like to reiterate and suggest scope for early detection and treatment by improving awareness among patients and physicians in India.
Lifestyle factors as tobacco smoking, heavy alcohol consumption, obesity and physical inactivity pose higher risk for PC. Tobacco smoking as the cause contributes substantially to the high mortality rates of PC.[56,57] Among non-modifiable risk factors, age >50 years, male gender have elevated risk.[58] Conditions like diabetes also increase the risk for PC, and India has a prevalence of 10% diabetes, which is associated with 75% of PCs.[59] Histology plays important role in prognosis and survival of PC. Around 85% of PC are adenocarcinomas and are most aggressive with very poor prognosis and, hence, have poorer survival unlike pancreatic neuro-endocrine cancer, nonadenocarcinomas, and squamous cell carcinomas forms. A causative role for pancreatitis in carcinoma of pancreas in humans has been strongly suggested based on various similar studies.[60] As PC remains silent symptomatically, early diagnosis is not possible in early stage and thus most of the cases are diagnosed in advanced stage.
The regional variations in cancer type occurrence across the globe indicated that socio-economic class and changes in various lifestyle habits have had a great impact on the outcome of cancer burden.[1] Data from various countries clearly show that more than approximately 40% of cancer could be eliminated by reducing modifying lifestyles and environmental factors.[61-63] Similarly, lipid-lowering statins have a modest role in decreasing risk of PC.[64] The data, especially with respect to India from HBCRs, on risk factors are not available would be considered as potential limitation of the study.
Despite the effective outcomes in prevention from various interventions, the health authorities and policy makers have not taken enough pro-active measures, probably due to the ignorance of prevention benefits and this has largely contributed to the increasing number of cases world over.[65]
PC is a rare cancer and is predicted to increase in future. The 5-year survival had shown an increase from 6% to 9% from year 2014 to 2018 pointing out toward the progress made in PC knowledge. But still, PC is among aggressive malignancies with the mortality and incidence ratio of 94%.[62,66] Comparison between the countries with very high/ high HDI and low HDI showed remarkable difference in the incidence and mortality rates though there was not much difference in the survival rates.[67]
CONCLUSION
Early detection and awareness holds key in improving outcomes. Since screening of large population may not be feasible, yet identifying high-risk groups, as those with family history, might be targeted for early detection. It is important to understand that the risk factors are very important for planning prevention strategies.
In low-resource countries with a low-HDI, the limitations of health infrastructure, lack of proper diagnostic tools may be a deterrent which will reflect on outcomes in terms of incidence and mortality. Prevention and lifestyle changes are the key to show improvements in PC patient’s outcomes, in terms of prognosis and survival.
More studies in India should be undertaken to address the issue of tobacco and lifestyle habits which play important role as factors in PC. However, studies should also address significant factors such as obesity, physical activity, and diet which is important to improve the survival of cancer patients. Strategies to modify lifestyle habits, increasing awareness through education, legislations, and laying more emphasis on prevention will be key for reduction in number of PC cases in the years to come.
ACKNOWLEDGMENTS
I would like to thank Dr. R A Badwe, Director and Dr. Shailesh Shrikhande, Deputy Director and Head of GI-DMG, for providing the support and encouragement. I would like to thank and acknowledge Mrs. Esha Dashmukhe for all her assistance in tabulation of the registry data and all the staff of registry for abstraction of cases from patient case records.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- International Agency for Research on Cancer, 2018. Available from: http://www.gco.iarc.fr [Last accessed on 2020 Jun 03]
- [Google Scholar]
- Cancer Incidence in Five Continents. In: Bray F, Colombet M, Mery L, Piñeros M, Znaor A, Zanetti R, eds. Vol. Vol 11. Lyon: International Agency for Research on Cancer; 2017. Available from: https://www.publications.iarc.fr/databases/iarc-cancerbases/cancer-incidence-in-five-continents-vol.-xi-2017 [Last accessed on 2020 Jun 03]
- [Google Scholar]
- Three-Year Report of Population Based Cancer Registries 2012-2014, Incidence, Distribution, Trends in Incidence Rates and Projections of Burden of Cancer (Report of 27 PBCRs in India), NCDIR-NCRP, ICMR. 2016. Available from: http://www.ncdirindia.org/ncrp/all_ncrp_reports/pbcr_report_2012_2014/all_content/pdf_printed_version/preliminary_pages_printed.pdf [Last accessed on 2020 Jun 03]
- [Google Scholar]
- Time Trends in Cancer Incidence Rates 1982-2010, NCDIR-NCRP, ICMR. 2013. Available from: https://www.icmr.nic.in/sites/default/files/reports/preliminary_pages6.pdf [Last accessed on 2020 Jun 03]
- [Google Scholar]
- American Cancer Society, Cancer Facts and Figures. 2014. Atlanta: American Cancer Society; Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2014 [Last accessed on 2020 Jun 03]
- [Google Scholar]
- More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. 2016;55:1158-60.
- [CrossRef] [PubMed] [Google Scholar]
- Consolidated Report of Hospital Based Cancer Registries: 2012-2014, NCDIR, ICMR. 2016a. Available from: https://www.icmr.nic.in/sites/default/files/reports/preliminary_pagesp.pdf [Last accessed on 2020 Jun 03]
- [Google Scholar]
- Hospital cancer registry In: Desai RB, Rao RS, Rao DN, Shroff PD, eds. Annual Report-1987. Mumbai: Tata Memorial Hospital; 1989.
- [Google Scholar]
- Hospital cancer registry In: Desai RB, Rao RS, Rao DN, Shroff PD, eds. Annual Report-1992. Mumbai: Tata Memorial Hospital; 1994.
- [Google Scholar]
- Hospital cancer registry In: Dinshaw KA, Rao DN, Ganesh B, eds. Annual Report-1997. Mumbai: Tata Memorial Hospital; 2001.
- [Google Scholar]
- Hospital cancer registry In: Dinshaw KA, Ganesh B, eds. Annual Report-2002-2005. Mumbai: Tata Memorial Hospital; 2008.
- [Google Scholar]
- Hospital cancer registry In: Badwe RA, DeCruz AK, Ganesh B, eds. Annual Report-2006-2008. Mumbai: Tata Memorial Hospital; 2015.
- [Google Scholar]
- Hospital cancer registry In: Badwe RA, DeCruz AK, Chiplunkar S, Ganesh B, eds. Annual Report-2012-2013. Mumbai: Tata Memorial Hospital; 2017.
- [Google Scholar]
- Hospital cancer registry In: Badwe RA, Pramesh CS, Ganesh B, eds. Annual Report-2017. Mumbai: Tata Memorial Hospital; 2019.
- [Google Scholar]
- Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-86.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical utility of the CA 19-9 tumour-associated antigen. Am J Gastroenterol. 1990;85:350-5.
- [Google Scholar]
- Haribhakti S, ed. Clinical GI Surgery. Ahmedabad: Haribhakti Education Foundation; 2008. p. :627-41.
- Multimodality imaging of pancreatic ductal adenocarcinoma: A review of the literature. HPB (Oxford). 2012;14:658-68.
- [CrossRef] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. 2019. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx#pancreatic [Last accessed on 2020 Jun 03]
- [Google Scholar]
- Pancreatic malignancies. In: Practical Clinical Oncology (1st ed., Ch. 34). New Delhi: Jaypee Brothers Medical Publishers; 2014.
- [CrossRef] [Google Scholar]
- EUROCARE-3: Survival of cancer patients diagnosed 1990-94-results and commentary. Ann Oncol. 2003;14(Suppl 5):v61-118.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (The international cancer benchmarking partnership): An analysis of population-based cancer registry data. Lancet. 2011;377:127-38.
- [CrossRef] [Google Scholar]
- SEER Cancer Statistics Review, 1975-2013. 2016. Bethesda. MD: National Cancer Institute; Available from: https://www.seer.cancer.gov/archive/csr/1975_2014 [Last accessed on 2020 Jun 03]
- [Google Scholar]
- Survival from cancer of the pancreas in England and Wales up to 2001. Br J Cancer. 2008;99(Suppl 1):S21-3.
- [CrossRef] [PubMed] [Google Scholar]
- Recent trends of cancer in Europe: A combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer. 2008;44:1345-89.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of pancreatic cancer in Northeastern Italy: Incidence resectability rate hospital stay, costs and survival (1990-1992) Dig Liver Dis. 2002;34:723-31.
- [CrossRef] [Google Scholar]
- Survival from common and rare cancers in Germany in the early 21st century. Ann Oncol. 2012;23:472-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer survival in Africa, Asia, the Caribbean and Central America. Introduction. IARC Sci Publ. 2011;162:1-5.
- [Google Scholar]
- Global patterns and trends in pancreatic cancer incidence: Age, period, and birth cohort analysis. Pancreas. 2019;48:199-208.
- [CrossRef] [PubMed] [Google Scholar]
- Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311:183-92.
- [CrossRef] [PubMed] [Google Scholar]
- A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med. 1996;156:2255-60.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for pancreatic cancer: Case-control study. Am J Gastroenterol. 2007;102:2696-707.
- [CrossRef] [PubMed] [Google Scholar]
- Association between alcohol intake and the risk of pancreatic cancer: A dose-response meta-analysis of cohort studies. BMC Cancer. 2016;16:212.
- [CrossRef] [PubMed] [Google Scholar]
- Comment on red and processed meat consumption and risk of pancreatic cancer: Meta-analysis of prospective studies. Br J Cancer. 2012;107:754-5.
- [CrossRef] [PubMed] [Google Scholar]
- Meat and meat-mutagen intake and pancreatic cancer risk in the NIH-AARP cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:2664-75.
- [CrossRef] [PubMed] [Google Scholar]
- Main dietary compounds and pancreatic cancer risk. The quantitative analysis of case-control and cohort studies. Cancer Epidemiol. 2012;36:60-7.
- [CrossRef] [PubMed] [Google Scholar]
- Meat and fish consumption and risk of pancreatic cancer: Results from the European prospective investigation into cancer and nutrition. Int J Cancer. 2013;132:617-24.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality in vegetarians and comparable non-vegetarians in the United Kingdom. Am J Clin Nutr. 2016;103:218-30.
- [CrossRef] [PubMed] [Google Scholar]
- The association between obesity and cancer risk: A meta-analysis of observational studies from 1985 to 2011. ISRN Prev Med. 2013;2013:680536.
- [CrossRef] [PubMed] [Google Scholar]
- New strategies in pancreatic cancer: Emerging epidemiological and therapeutic concepts. Clin Cancer Res. 2010;16:4313-8.
- [CrossRef] [PubMed] [Google Scholar]
- Are racial disparities in pancreatic cancer explained by smoking and overweight/obesity? Cancer Epidemiol Biomarkers Prev. 2009;18:2397-405.
- [CrossRef] [PubMed] [Google Scholar]
- Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett. 2016;381:269-77.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreatic cancer in England and Wales 1975-2000. Patterns and trends in incidence, survival and mortality. Aliment Pharmacol Ther. 2006;23:1205-14.
- [CrossRef] [PubMed] [Google Scholar]
- Reproductive factors and risk of pancreatic cancer in women: A review of the literature. Ann Epidemiol. 2009;19:103-11.
- [CrossRef] [PubMed] [Google Scholar]
- Menstrual and reproductive factors and pancreatic cancer in the SEARCH program of the IARC. Cancer Causes Control. 2009;20:1757-62.
- [CrossRef] [PubMed] [Google Scholar]
- SEER Cancer Statistics Review, 1975-2005. 2008. Based on November 2007, SEER Data Submission. Posted to the SEER Web Site. Bethesda, MD: National Cancer Institute; Available from: https://www.seer.cancer.gov/archive/csr/1975_2005 [Last accessed on 2020 Jun 03]
- [Google Scholar]
- Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634-8.
- [CrossRef] [PubMed] [Google Scholar]
- Anticipation in familial pancreatic cancer. GUT. 2006;55:252-8.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: Deleterious BRCA2 mutations in 17%. Cancer Res. 2002;62:3789-93.
- [Google Scholar]
- Population attributable risk for pancreatic cancer in Northern Italy. Pancreas. 2015;44:216-20.
- [CrossRef] [PubMed] [Google Scholar]
- Association of diabetes mellitus and pancreatic adenocarcinoma: A meta-analysis of 88 studies. Ann Surg Oncol. 2014;21:2453-62.
- [CrossRef] [PubMed] [Google Scholar]
- Is diabetes mellitus a risk factor for pancreatic cancer? World J Gastroenterol. 2013;19:4861-6.
- [CrossRef] [PubMed] [Google Scholar]
- Type 2 diabetes mellitus and pancreatic cancer. Surg Clin North Am. 2013;93:619-27.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetes, smoking, alcohol use, and family history of cancer as risk factors for pancreatic neuroendocrine tumors: A systematic review and meta-analysis. Neuroendocrinology. 2015;101:133-42.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of pancreatic cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10-27.
- [CrossRef] [PubMed] [Google Scholar]
- Role of smoking in global and regional cancer epidemiology: Current patterns and data needs. Int J Cancer. 2005;116:963-71.
- [CrossRef] [PubMed] [Google Scholar]
- The temporal relation between cigarette smoking and pancreatic cancer. Am J Public Health. 1983;73:1403-4.
- [CrossRef] [PubMed] [Google Scholar]
- The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252-61.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of pancreatic carcinoma in tropical calcifying pancreatitis: An epidemiologic study. Pancreas. 1994;9:62-6.
- [CrossRef] [PubMed] [Google Scholar]
- Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68:31-54.
- [CrossRef] [PubMed] [Google Scholar]
- How many cancer cases and deaths are potentially preventable? Estimates for Australia in 2013. Int J Cancer. 2018;142:691-701.
- [CrossRef] [PubMed] [Google Scholar]
- The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br J Cancer. 2018;118:1130-41.
- [CrossRef] [PubMed] [Google Scholar]
- Statins reduce the risk of pancreatic cancer in humans: A case-control study of half a million veterans. Pancreas. 2007;34:260-5.
- [CrossRef] [PubMed] [Google Scholar]
- Framework for understanding cancer prevention. Cancer Epidemiology and Prevention 2018:1193-204.
- [CrossRef] [Google Scholar]
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- [CrossRef] [PubMed] [Google Scholar]
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- [CrossRef] [PubMed] [Google Scholar]






