Translate this page into:
Comparative evaluation of broth microdilution with E-test, Vitek 2, and disk diffusion for susceptibility testing of colistin on Gram-negative bacteria

*Corresponding author: Parul Gupta, Department of Microbiology, SMS Medical College and Hospital, Jaipur - 302 004, Rajasthan, India. drrajnisharma2@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gupta P, Sharma R, Vyas A, Tak A. Comparative evaluation of broth microdilution with E-test, Vitek 2, and disk diffusion for susceptibility testing of colistin on Gram-negative bacteria. Indian J Med Sci 2021;73(1):93-8.
Abstract
Objectives:
With the increasing threat of multidrug-resistant organisms, colistin has become popular in clinical practice. A better understanding of antimicrobial susceptibility testing methods for colistin is needed for optimal patient management. The aim of the study was to determine the accuracy of E-test, Vitek 2 system for the detection of colistin minimum inhibitory concentrations (MIC) against broth microdilution (BMD).
Material and Methods:
A total of 100 isolates of Gram-negative bacilli were subjected to susceptibility testing for colistin using the following methods: BMD, E-test, Vitek 2, and disk diffusion. Using BMD as the gold standard, comparative analysis between different methods was carried out.
Results:
Comparison of MIC values of E-test (GM = 0.488 mg/ml) against BMD (GM = 0.611 mg/ml using unpaired t-test (t = 2.015, P = 0.045) showed that geometric means of MIC values of E-strip were significantly lower than BMD. Similarly, comparison of MIC values of Vitek 2 system (GM = 0.615 mg/ml) against BMD (GM = 0.611 mg/ml) using unpaired t-test (t = −0.050, P = 0.960) showed no statistical significant differences in geometric means of MIC values. Taking reference as BMD method – the EA for E-strip is 57%, CA is 97%, VME is 2%, and no ME. Similarly, for the Vitek method EA is 64%, CA is 98%, VME is 1%, and ME is 1%.
Conclusion:
Different susceptibility testing methods for colistin show great variation in their results and BMD is the best candidate as gold standard. The Vitek 2 method showed good concordance with BMD.
Keywords
Broth microdilution
Colistin
E-test
Vitek 2
INTRODUCTION
Polymyxins are a novel class of antimicrobials that (due to their cationic cyclic polypeptide moiety) have a surface-active detergent-like action against most Gram-negative bacteria (GNB), including multidrug-resistant (MDR) pathogens.[1] The main representatives of polymyxins utilized in clinical practice are polymyxin B and polymyxin E (colistin). Colistin and polymyxin B are wont to treat infections caused by GNB that are immune to aminoglycosides, cephalosporins, anti-Pseudomonas penicillins, quinolones, monobactams, and carbapenems.[2,3] Thus, they are getting used as a final resort drug for the treatment of life-threatening infections. Colistin was a well-liked antimicrobial agent from 1960s to 1980s, but fell into disrepute due to serious nephro- and neurotoxicity.[4] Strains of Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae that exhibit resistance to most available antibiotics except polymyxins, have emerged as a standard explanation for hospital acquired infections in critically ill patients.[5,6] Living within the “era of antibiotic resistance” physicians within the last decade has revived use of colistin, since the available literature at the clinical level was poor and limited.[7] As a consequence, the emergence of colistin-resistant bacteria, although reported infrequently till date,[8] is becoming a clinical concern. The in vitro susceptibility testing of polymyxin group antimicrobials is hampered by several various factors. The accuracy of the disk diffusion assay is unsatisfactory because polymyxins diffuse poorly into agar, and consequently no reliable correlation of zone diameters and minimum inhibitory concentrations (MICs) has been found in some studies.[9] The interpretative criteria for quantitative in vitro testing also differ between nations.[9] The Clinical and Laboratory Standards Institute (CLSI) approved a typical document for the testing of polymyxins against P. aeruginosa, Acinetobacter spp., and a couple of other non-fermenters using dilution methods.[10] It had been only in 2007 that the interpretative criteria for disk susceptibility testing of colistin were published by the CLSI.[10] However, there is still no consensus regarding the breakpoints for outlining resistance to colistin. Since relatively few surveys of antibiotic resistance are performed on this group of antimicrobials, reliable data on true resistance levels also are lacking. Considering the increasing use and demand for colistin and therefore the relative paucity of knowledge regarding resistance, we evaluated different susceptibility testing methods for this class of antimicrobial within the present study. The present study was planned to systemically evaluate the varied methods of susceptibility testing available for colistin.
MATERIAL AND METHODS
This study was conducted in the Department of Microbiology Sawai Man Singh Medical College (SMS) Jaipur (Rajasthan) from July 2018 to July 2019. Hundred strains of GNB isolated from clinical samples, i.e., blood, sputum, pus, urine, and tracheal aspirate were included in the study. Identification up to species level was done by conventional methods as per laboratory SOP.[11] Antibiotic susceptibility testing was performed by Kirby–Bauer disk diffusion method as per CLSI guidelines.[12] Escherichia coli ATCC 25922 and P. aeruginosa ATCC 27853 were used as control strains for susceptibility testing.[13]
AST methods
All 100 isolates were evaluated for colistin susceptibility by four different methods: Broth microdilution (BMD) (colistin sulfate, 15,000 IU/mg; HIMEDIA, Mumbai, India), disk diffusion (DD) (HiMedia, Mumbai, India), E-test (0.016– 256 mg/ml BioMerieux), and Vitek 2 (BioMerieux, Marcy l’Etoile, France). For BMD, two-fold drug dilutions ranging from 16 to 0.125 mg/ml were prepared for colistin and the test was performed according to CLSI guidelines.[13] The isolates were subjected to DD using colistin 10 mg disk. E-test was also carried out for all the isolates. The isolates were also subjected to susceptibility analysis with Vitek 2 using the AST-N281 and AST-N280 card following the manufacturer’s instructions. Control strains were evaluated with each technique and BMD was performed in duplicate to ensure repeatability and reproducibility, as directed by CLSI.[14]
Interpretation of AST methods
The recommended MIC breakpoints for colistin were used for result analysis. For Acinetobacter spp., CLSI and EUCAST recommend a breakpoint of ≤2 mg/ml for susceptible and >4 mg/ml for resistant isolates. MIC for ATCC strains of 0.25–2 mg/ml was taken for E. coli and 0.5–4 mg/ml for P. aeruginosa. For E-test, MICs of intermediate values were rounded off to the next doubling dilution for comparison. Since CLSI and EUCAST do not recommend that zone diameter breakpoints for colistin for DD zone diameters of ≤12 and ≥9 mm, as proposed by Piewngam and Kiratisin[15] were used as reference.
Analysis of data
The MIC results obtained by different methods were analyzed by comparing against the MIC obtained by BMD. Essential agreement (EA) was calculated as the percentage of isolates that had MIC values within one two-fold dilution of the reference standard. Categorical agreement (CA) was calculated as the percentage of isolates with results in the same category as the reference standard, taking all isolates tested as the denominator. Very major error (VME; an isolate resistant by the reference method, but susceptible by the test method) denoted false susceptibility, while major error (ME; an isolate susceptible by the reference method, but resistant by the test method) denoted false resistance. Minor error, defined as the isolate being resistant or susceptible by the reference method, but intermediate by the test method, was obviated as there is no intermediate susceptibility category for colistin. Reliability of individual tests in delineating breakpoints was determined according to the following criteria: High if both EA and CA was >90%, moderate if either EA or CA is >90%, low if both EA and CA was <90%, and there were acceptable errors (<2% VME, <5% ME), and poor if the errors were unacceptable, irrespective of EA and CA. As two-fold dilution of MIC was taken, the comparison was done using difference of geometric means. Hence, first, the MIC values are transformed into the logarithmic with base 2 data. The geometric means were calculated using the formula given below:[16,17]
The significance test was designed for comparing the difference of arithmetic means of logarithmic MIC values. The two-tailed unpaired t-test at 5% level of significance was applied.
RESULTS
Total 100 clinical isolates of GNB were included in the study which was from blood (27%), urine (24%), and pus (23%) sample, followed by sputum (14%) and tracheal aspirate (12%). Among these isolates, 55% were K. pneumonia followed by E. coli (23%), A. baumannii (11%), and P. aeruginosa (6%). Out of 100 isolates of GNB 24% were found to be MDR. These MDR isolates were resistant to three and more classes of antibiotics which include penicillin, third-generation cephalosporins, aminoglycosides, and carbapenems. These isolates include K. pneumoniae 11%, E. coli 2%, A. baumannii 7%, P. aeruginosa 2%, and Enterobacter aerogenes 2%.
Results of individual AST for colistin
With BMD out of 100 isolates, 95 isolates were sensitive to colistin. Among the sensitive isolates 52% and 24% gave MIC of 0.5 mg/ml and 1 mg/ml, respectively. Out of five resistant isolates, two isolates have MIC of 4 mg/ml while three isolates have MIC of 8 mg/ml. Resistant isolates include two K. pneumonia, one A. baumannii, and two P. aeruginosa. The geometrical mean was 0.611 mg/ml by BMD.
Comparison of MIC values of E-strip susceptibility test (GM = 0.488 mg/ml) against the gold standard BMD (GM = 0.611 mg/ml) using unpaired t-test (t = 2.015, P = 0.045) showed that geometric means of MIC values of E-strip were significantly lower than BMD [Figure 1]. With E-test 97 isolates were sensitive with the MIC ranging between 0.06 and 1 mg/ml (1.5 mg/ml rounded off to next highest dilution). All three resistant isolates have MIC of 4 mg/ ml [Figure 2a]. These resistant isolates included one A. baumannii and two P. aeruginosa.
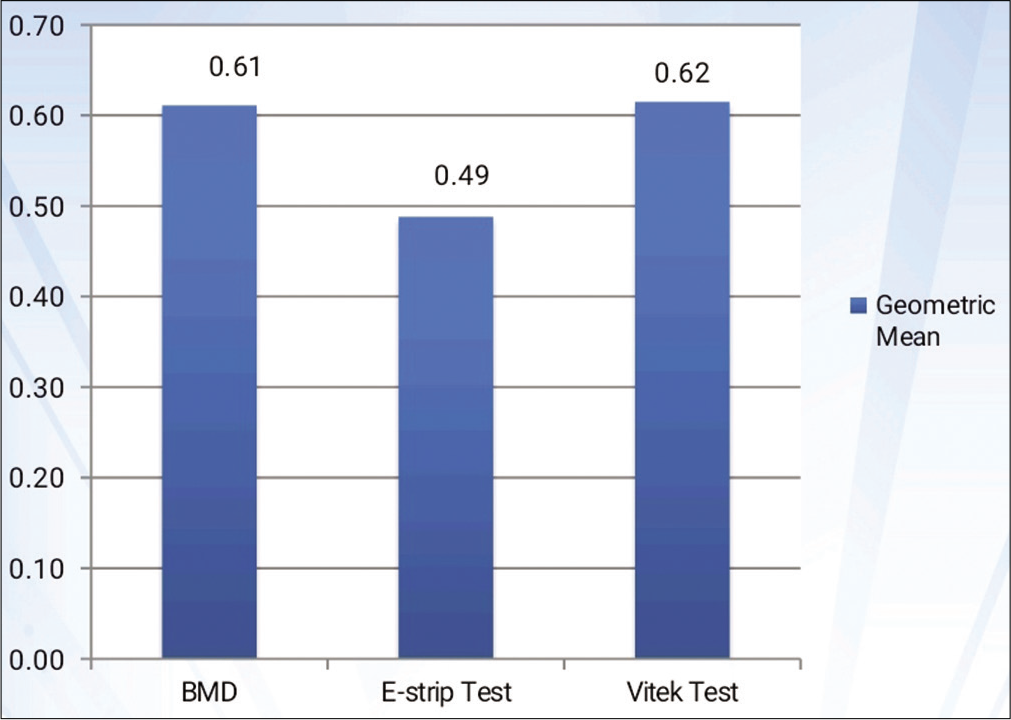
- Comparison of geometrical mean of various test methods to reference method. *P value on comparing BMD and E-strip test. #P value on comparing BMD Vitek 2 test.

- (a and b) Scatter diagram showing the result of MICs of colistin with Y-axis showing the MIC range of the reference method and the X-axis showing the MIC range of the test method. The MIC cutoffs for colistin are demarcated with solid lines. Very major are depicted by square and major error by circles.
With Vitek 2 system, 95 isolates were found sensitive. About 68% and 20% isolates have MIC value 0.5 mg/ml and 1 mg/ml, respectively. Out of five resistant isolates, two had MIC of 8 mg/ml and three isolates had MIC of 16 mg/ml [Figure 2b]. These included two K. pneumonia, two A. baumannii, and one P. aeruginosa. Comparison of MIC values of Vitek susceptibility test (GM = 0.615 mg/ml) against the gold standard BMD (GM = 0.611 mg/ml) using unpaired t-test (t = −0.050, P = 0.960) showed no statistical significant differences in geometric means of MIC values.
With DD 91 isolates were sensitive with zone size diameter >12 mm. Resistant isolates included five K. pneumonia, one A. baumannii, and three P. aeruginosa.
Comparison of different MIC testing methods for colistin susceptibility
Table 1 is showing comparision of results of all tests. Taking BMD as reference method [Table 2] – the EA for colistin for E-strip is 57%, CA is 97%, VME is 2%, and no ME. On interpretation, the reliability is moderate. Similarly, for the Vitek method EA is 64%, CA is 98%, VME is 1%, and ME is 1%. The reliability of Vitek method is also moderate.
| Species | Number of isolates with BMD MIC (mcg/ml) | Number of isolates with E-test results MIC (mcg/ml) | Number of isolates with Vitek 2 results MIC (mcg/ml) | Number of isolates with disc diffusion results | ||||
|---|---|---|---|---|---|---|---|---|
| ≤2 | ≥4 | ≤2 | ≥4 | ≤2 | ≥4 | S* | R# | |
| Klebsiella pneumonia (55) | 53 | 2 | 55 | 0 | 53 | 2 | 50 | 5 |
| Escherichia coli (25) | 25 | 0 | 25 | 0 | 25 | 0 | 25 | 0 |
| Acinetobacter baumannii (11) | 10 | 1 | 10 | 1 | 9 | 2 | 10 | 1 |
| Pseudomonas aeruginosa (6) | 4 | 2 | 4 | 2 | 5 | 1 | 3 | 3 |
| Enterobacter aerogenes (3) | 3 | 0 | 3 | 0 | 3 | 0 | 3 | 0 |
| Test | CA (%) | EA (%) | VME (%) | ME (%) |
|---|---|---|---|---|
| BMD and DD | 91 | -* | 0 | 4 |
| BMD and E-test | 97 | 57 | 2 | 0 |
| BMD and Vitek 2 | 98 | 64 | 1 | 1 |
DISCUSSION
The emerging MDR in nosocomial GNB has necessitated the use of parenteral polymyxins for the treatment of life-threatening infections. Therefore, there is an increased need for reliable susceptibility testing methods to predict clinical response. Susceptibility testing for colistin is plagued with problems, such as the lack of consensus regarding breakpoints for resistance between the CLSI and EUCAST; the reported poor diffusion of colistin in the agar; and the lack of correlation between different dilution methods, as well as lacunae in studies done on this group of antimicrobials, most of which have been done using colistin.[18-20] In this study, we evaluated colistin MIC’s obtained by three test methods for 100 GNB isolates from various clinical samples at our hospital. MIC’s obtained with BMD were used as the reference.
The DD method of AST has been rather unpopular for polymyxins. In the present study, DD for colistin was carried out using Piewngam’s criteria.[15] Ninety-one isolates produced zone diameters in the susceptible range, thus giving a CA of 91% with BMD. Since exact MIC values could not be interpreted with DD, evaluation of EA was not possible. VME with DD in our study was 0% while ME was 4%. By DD false resistance was found in three isolates of K. pneumoniae and one isolate of Pseudomonas. Studies have reported VMEs ranging from 1% to 3.5%[21,22] for the colistin with low agreement when compared with BMD. The high value of ME in the current study showed that DD gives false resistance and it is not a reliable method to use routinely in replace of BMD. Studies conducted by Behera et al.[21] and Hijden et al.[19] showed 91.2% and 88.7% susceptibility to colistin, respectively, which are comparable to the results in our study.
In the present study, CA with E-test was 97% and EA was 57%. There was no ME with E-test while VME was 2%. This denotes it falsely identified two isolates of Klebsiella as sensitive which was resistant by BMD. These values are in agreement with the study by Arroyo et al.[22] who have reported EA and CA of 16.5% and Singhal et al.[23] who reported EA 16.6% and CA >90% and 98.2%, respectively. The highest rate of VMEs reported for colistin after an E-test is 41.5%.[24] Such observations could be attributed to different brands of E-strips used, thus hindering effective drug penetration through the agar medium. E-test is currently not recommended as a testing method for colistin MIC.[24,25]
In the current study with Vitek 2 automated system, five isolates of GNB were resistant with MIC values ≥4 mg/ml resistant as per manufacturer’s guidelines With comparison to BMD method, Vitek 2 failed to detect one resistant isolate of Acinetobacter sp and one isolate of Pseudomonas sp.
With Vitek 2 EA and CA were 64% and 98%, respectively, as compared to BMD. There was only 1% VME and 1% ME. The CA (98%) was good enough, contributing to an overall moderate reliability of Vitek 2. This is in agreement with two prior studies that observed moderate agreement of Vitek with BMD despite CA of 94% and 90%,[19,22] respectively. Vitek 2 was previously reported as a good testing method for colistin,[24] same was reproduced in our study. With Vitek 2 high CA and EA gave moderately good reliability as compared to BMD. Those isolates are resistant with Vitek 2 need further confirmation with BMD.
CONCLUSION
There are no new antibiotics against Gram-negative organisms in the pipeline therefore colistin, the last resort drug should be preserved and used judiciously after antibiotic susceptibility testing and following antibiotic stewardship. Disk diffusion method should not be used as routine testing method for colistin sensitivity as it gives most inconsistent results as compared to the reference standard method due to poor diffusibility of colistin into the medium. E-test is less reliable for colistin susceptibility due to considerably lower CA for colistin. It failed to identify all resistant strains.
Vitek 2 automated system gave consistent results with the reference standard method and can be used routinely for colistin MIC testing but the resistant subpopulations should be rechecked with BMD. Microbroth dilution is the most sensitive, reliable, and cost-effective method for colistin susceptibility testing. Only disadvantage is that it is a time-consuming procedure and trained personal is required to perform the test. Therefore, alternative methods such as E strip test and Vitek 2 automated system can be used. Further studies are required to identify the gene/s responsible for resistance and simple screening method to detect colistin resistance.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Polymyxin B sulfate and colistin: Old antibiotics for emerging multiresistant gram-negative bacteria. Ann Pharmacother. 1999;33:960-7.
- [CrossRef] [PubMed] [Google Scholar]
- Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin Infect Dis. 1999;28:1008-11.
- [CrossRef] [PubMed] [Google Scholar]
- Colistin: An update on the antibiotic of the 21st century. Expert Rev Anti Infect Ther. 2012;10:917-34.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome of infections due to pandrug-resistant (PDR) gram-negative bacteria. BMC Infect Dis. 2005;5:24.
- [CrossRef] [PubMed] [Google Scholar]
- Colistin treatment in patients with ICU-acquired infections caused by multiresistant gram-negative bacteria: The renaissance of an old antibiotic. Clin Microbiol Infect. 2005;11:115-21.
- [CrossRef] [PubMed] [Google Scholar]
- How should we respond to the emergence of plasmid-mediated colistin resistance in humans and animals? Int J Infect Dis. 2017;54:77-84.
- [CrossRef] [PubMed] [Google Scholar]
- Bad bugs need drugs: An update on the development pipeline from the antimicrobial availability task force of the infectious diseases society of America. Clin Infect Dis. 2006;42:657-68.
- [CrossRef] [PubMed] [Google Scholar]
- Pitfalls of polymyxin antimicrobial susceptibility testing of Pseudomonas aeruginosa isolated from cystic fibrosis patients. J Antimicrob Chemother. 2004;54:1057-61.
- [CrossRef] [PubMed] [Google Scholar]
- Performance Standards for Antimicrobial Susceptibility Testing Seventh Informational Supplement M100-S15 Wayne, PA: Clinical and Laboratory Standards Institute; 2005.
- [Google Scholar]
- Performance Standards for Antimicrobial Susceptibility Testing Seventh Informational Supplement M100-S17 Wayne, PA: Clinical and Laboratory Standards Institute; 2007.
- [Google Scholar]
- Breakpoint Tables for Interpretation of MICs and Zone Diameters. 2017. European Committee on Antimicrobial Susceptibility Testing. Available from: http://www.eucast.org [Last accessed on 2019 Dec 15]
- [Google Scholar]
- Performance Standards for Antimicrobial Susceptibility Testing In: CLSI Supplement M100 (27th ed). Wayne, PA: Clinical and Laboratory Standards Institute; 2017.
- [Google Scholar]
- Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard In: CLSI Document M07-A9 (9th ed). Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
- [Google Scholar]
- Comparative assessment of antimicrobial susceptibility testing for tigecycline and colistin against Acinetobacter baumannii clinical isolates, including multidrug-resistant isolates. Int J Antimicrob Agents. 2014;44:396-401.
- [CrossRef] [PubMed] [Google Scholar]
- The geometric mean: Confidence limits and significance tests. Percept Psychophys. 1979;26:419-21.
- [CrossRef] [Google Scholar]
- Comparison of Etest, Vitek and agar dilution for susceptibility testing of colistin. Clin Microbiol Infect. 2007;13:541-4.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of disc diffusion, Etest and broth microdilution for testing susceptibility of carbapenem-resistant Pseudomonas aeruginosa to polymyxins. Ann Clin Microbiol Antimicrob. 2007;6:8.
- [CrossRef] [PubMed] [Google Scholar]
- Polymyxin antibiotics for gram-negative infections. Am J Health Syst Pharm. 2007;64:819-26.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of susceptibility testing methods for polymyxin. Int J Infect Dis. 2010;14:e596-601.
- [CrossRef] [PubMed] [Google Scholar]
- Reliability of the E-test method for detection of colistin resistance in clinical isolates of Acinetobacter baumannii. J Clin Microbiol. 2005;43:903-5.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative evaluation of broth microdilution with polystyrene and glass-coated plates, agar dilution, E-Test, Vitek, and disk diffusion for susceptibility testing of colistin and polymyxin B on carbapenem-resistant clinical isolates of Acinetobacter baumannii. Microb Drug Resist. 2018;24:1082-8.
- [CrossRef] [PubMed] [Google Scholar]
- Recommendations for MIC Determination of Colistin (Polymyxin E) as Recommended by the Joint CLSI-EUCAST Polymyxin Breakpoints Working Group. 2016. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf [Last accessed on 2019 Dec 15]
- [Google Scholar]
- Comparative evaluation of colistin susceptibility testing methods among carbapenem-nonsusceptible Klebsiella pneumoniae and Acinetobacter baumannii clinical isolates. Antimicrob Agents Chemother. 2015;59:4625-30.
- [CrossRef] [PubMed] [Google Scholar]






