Translate this page into:
Does the size of unifocal papillary thyroid carcinomas affect the status of the cervical lymph nodes?
*Corresponding author: Emad Mofid Nassif Rezkallah, Department of General Surgery, South Tees Hospitals National Health Service (NHS) Foundation Trust, Middlesbrough, United Kingdom. emad.mofid@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Rezkallah EM, Mahmoud Y, Mekhaeil K, Hanna RS. Does the size of unifocal papillary thyroid carcinomas affect the status of the cervical lymph nodes?. Indian J Med Sci. 2024;76:7-11. doi: 10.25259/IJMS_151_2023
Abstract
Objectives:
Papillary thyroid carcinoma (PTC) is the most common endocrine malignancy in humans. Cervical lymph node (LN) involvement is one of the major prognostic factors in disease recurrence and morbidity. Despite central lymph node dissection (CLND) is recommended in the case of involved LNs, prophylactic neck dissection is still controversial due to the potential complications associated with this procedure. The aim of the current review is to assess the correlation between the sizes of unifocal PTC with cervical LN involvement, which could help to choose the best treatment plan for patients with PTC.
Materials and Methods:
We performed a retrospective review for all patients who had unifocal PTC in our department from 2013 to 2019 with a minimum of 3 years of follow-up. SPSS software was used to calculate this correlation.
Results:
Fifty-nine patients (38 females and 21 males) were included in our study with an average age of 45.4 ± 17 years of age. Out of 17 patients (28.8%) with microcarcinomas, six of them (10.2%) had cervical LN involvement, whereas of the 42 patients (71.2%) with macrocarcinomas, 17 of them (28.8%) had cervical LN metastasis. The correlation between the tumor size and the number of metastatic LNs in our study was weakly positive (r = 0.332, P < 0.05).
Conclusion:
The decision regarding CLND should be selected on an individual base as even small micro PTC could metastasize to the local LNs.
Keywords
Papillary thyroid carcinoma
Size
Lymph node
Metastasis
Correlation
INTRODUCTION
Thyroid carcinoma is the most common endocrine malignancy in humans. The four main subtypes of thyroid carcinoma are papillary thyroid carcinoma (PTC), medullary thyroid carcinoma, follicular thyroid carcinoma, and anaplastic thyroid carcinoma.[1] PTC makes up almost 90% of all thyroid carcinomas, making it the most common pathological subtype. 80–95% of PTC patients survive for at least 10 years.[2] Age, gender, tumor size, bilaterality, multifocality, extracapsular invasion, and angiolymphatic invasion are a few factors that may affect the prognosis of the disease. Young people with early lymph node (LN) metastasis frequently develop PTC.[3] The risk of developing cervical LN involvement in PTC patients ranges from 40% to 90%.[4] The central compartment is where LN metastasis first develops, then it spreads to the lateral compartment.[5] In addition, cervical LN metastasis is linked to a higher risk of mortality and recurrence in PTC patients.[6]
When evaluating the cervical LN status in PTC patients, high-resolution ultrasonography (US) is frequently used, with sensitivity and specificity reaching 97% and 93%, respectively.[7] Compared to the lateral neck compartment, deep central LNs of the retropharynx and mediastinum are less accurately assessed by ultrasound.[8] Many surgeons choose to perform prophylactic central lymph node dissection (CLND) due to the high false negative rate. However, not all surgeons have accepted routine prophylactic CLND because of the complications connected with the procedure.
The current study aims to assess the correlation between the size of unifocal PTCs and the status of the cervical LNs, which would help in future planning of the best surgical procedure for patients with PTC.
MATERIALS AND METHODS
We did a retrospective review for all patients who had PTC in our hospital and had surgical intervention during the period from 2013 to 2019 with a minimum of 3 years of follow-up after surgery. Patients’ data were collected from the hospital recording system. Our inclusion criteria were; (1) patients who had unifocal PTC of any age group, (2) had a surgical intervention with or without cervical LN dissection, and (3) had at least 3 years of follow-up after surgery. Exclusion criteria included: (1) patients who had multifocal PTC, (2) patients who had recurrence of previously treated PTC, and (3) other pathological subtypes of thyroid malignancy.
All patients had preoperative imaging (US ± computed tomography [CT] scan). Patients with suspicious imaging had fine-needle aspiration for cytology. All patients with confirmed or suspicious PTC were discussed in our endocrine multidisciplinary team meeting. Informed consent was obtained from every patient before any surgical intervention. Patients with aggressive histology, extrathyroidal extension, incomplete resection, pathologically positive LNs with at least one node ≥3 cm in the largest dimension, and distant metastasis all had radioactive iodine ablation after surgery. That was followed by thyroid stimulating hormone (TSH) suppression using levothyroxine.
We followed up with all patients for a minimum of 3 years to assess for any recurrence, especially in patients with negative LNs on initial surgery.
We calculated the correlation coefficient between the numbers of involved cervical LN with the tumor size using SPSS software version 25.
RESULTS
Fifty-nine patients (38 females and 21 males) were included in our study after applying the inclusion and exclusion criteria. The average age was 45.4 ± 17 years of age. Fifty-three patients had a total thyroidectomy, 16 patients underwent total thyroidectomy only, and 20 had a total thyroidectomy and central neck level VI dissection, whereas 17 patients had cervical LN involvement on preoperative investigations and underwent total thyroidectomy with central and lateral neck dissection. Six patients had lobectomy only. The average tumor size was 22 mm ± 17 mm. Seventeen patients (28.8%) had micro PTC (<1 cm) and eight of them (13.6%) had an incidental finding of the tumor. All patients had radioactive iodine ablation except 17 patients (28.8%) who had a completely excised microcarcinoma [Table 1].
| Variable | Value | Percentage |
|---|---|---|
| Age | 45.4±17 year of age | - |
| Gender | ||
| Males | 21 | 35.6 |
| Females | 38 | 64.4 |
| Type of surgery | ||
| Lobectomy | 6 | 10.2 |
| Total thyroidectomy | 16 | 27.1 |
| Total thyroidectomy and central dissection | 20 | 33.9 |
| Total thyroidectomy with central and lateral neck dissection | 17 | 28.8 |
| Tumor size | ||
| Average | 22±17 mm | - |
| No. of patients with microcarcinomas | 17 | 28.8 |
| No. of patients with macrocarcinomas | 42 | 71.2 |
| Lymph node metastasis | ||
| Average number | 7.4 LN | - |
| Of microcarcinomas | 6/17 | (10.2/28.8) |
| Of macrocarcinomas | 17/42 | (28.8/71.2) |
Of the 59 patients in our study, 36 patients (61.1%) had no LN involvement, whereas 23 patients (38.9%) had cervical LN metastasis. Of the 23 patients, 18 patients (30.4%) were under the age of 50, whereas 5 patients (8.5%) were over 50 years of age. Four patients had preoperative negative scans for any abnormal LN and had an incidental finding of positive LNs on the final histology. The correlation between the number of metastatic LNs with the age of the patients was negative (r = −0.154, P = 0.2), [Figure 1].

- Simple scatter with a fit line of the number of metastatic lymph nodes by the age of patients.
The average number of LN involvement was 7.4 LNs. Of the 17 patients (28.8%) with microcarcinomas, six patients (10.1%) had cervical LN involvement, whereas of the 42 patients (71.2%) with macrocarcinomas and 17 patients (28.8%) had cervical LN metastasis. All patients were followed up for a minimum of 3 years. Three patients only had a recurrence in our study; the three cases had cervical LN involvement during the initial surgery. The correlation coefficient between the tumor size and the number of metastatic LNs in our study was weakly positive (r = 0.332, P < 0.05), [Figure 2].

- Simple scatter with a fit line of the number of metastatic lymph nodes by the size of the tumor.
DISCUSSION
The most typical differentiated thyroid malignancy is PTC. Even though PTC has a very high survival rate, some patients still experience recurrences that could be fatal.[9] Young adults with early LN metastases are more likely to develop PTC than older adults.[3] Between 20% and 50% of PTC, cases may have cervical LN involvement.[6,10] An independent risk factor for recurrence is the existence of neck LN metastases.[11,12] PTC typically spreads to the lateral compartment after first starting to metastasize in the central compartment.[13] Central LN metastasis was mentioned in numerous studies as having the potential to raise the risk of recurrence, but it had no impact on overall survival.[4]
Although it is reasonable to perform therapeutic CLND, the decision for prophylactic dissection is still controversial due to the associated comorbidities; the risk of damage to the recurrent laryngeal nerve and parathyroid glands is of the common complications associated with central neck dissection.[14,15] To maximize the benefits to the patients, the decision regarding prophylactic neck dissection should be selected on an individual basis to the high-risk group patients rather than a single treatment therapy for all patients.
US, CT, magnetic resonance imaging, and positron emission tomography (PET), including [F18] F-fluorodeoxyglucose PET/CT, are all examples of preoperative diagnostic imaging.[16,17] In addition, fine-needle aspiration for cytology is possible with a neck US scan. The sensitivity rate of US and CT for diagnosing lateral LN metastasis was reported as 64.0–74.3% and 68.6–78.2%, respectively, and the specificity of both techniques was 82–94.8% and 78–95%, respectively,[18,19] [Figures 3 and 4]. On the other hand, preoperative CT and US are not very sensitive in assessing the central neck compartment.[20] Consequently, it is frequently challenging to make an accurate preoperative assessment of the central neck compartment. 59 patients with unifocal PTC were included in our study, 23 (38.9%) of them had cervical LN involvement. Four patients of the 23 had incidental findings of central LN involvement in the presence of negative preoperative imaging.
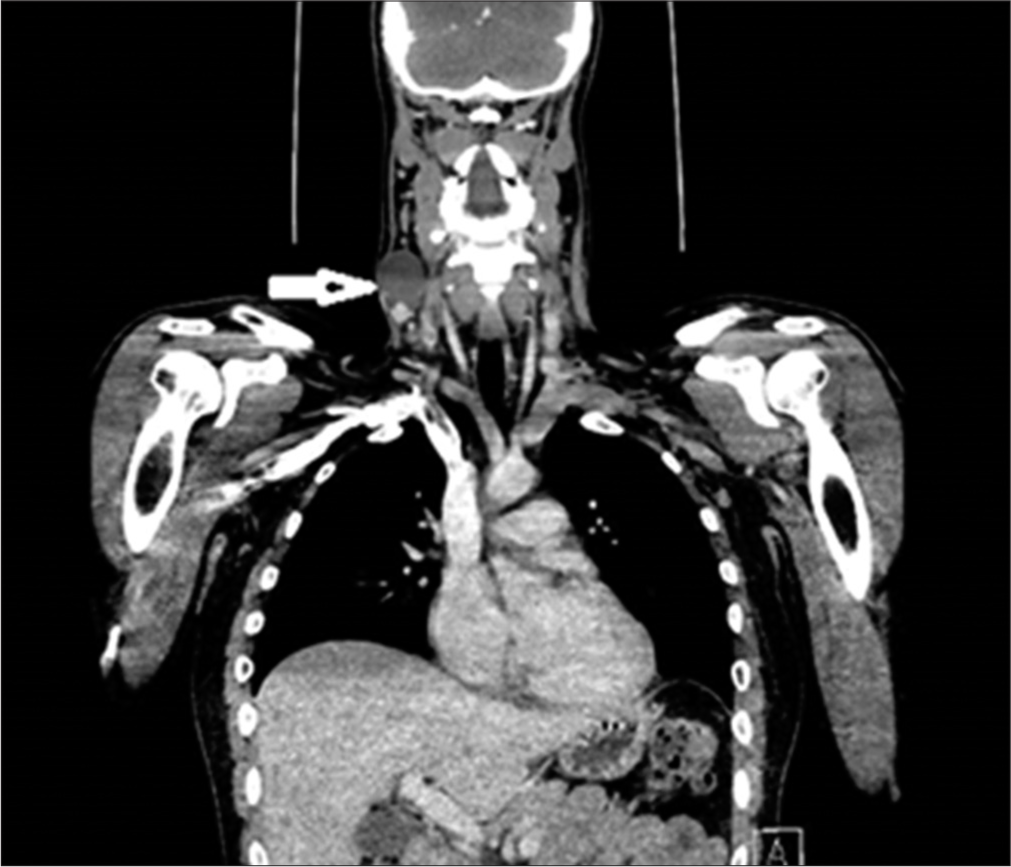
- Computed tomography of neck and thorax showing cystic metastatic necrotic lymph node on the right side of the neck at level IV (white arrow) showing an enhancing component inferiorly with the largest superior inferior dimension of 41 mm. This abuts but does not involve the internal jugular vein or the common carotid artery.

- Neck ultrasonography scan showing multiple enlarged, heterogeneous, level II–VI left neck lymph nodes (white arrow) with disorganized vascularity measuring up to 24 mm. Metastasis was confirmed on fine-needle aspiration cytology.
With regard to age, age was considered a major factor in staging differentiated thyroid carcinomas (DTCs). Many studies reported that age < 45 years could be used as an independent predictor of LN involvement in DTC.[21,22] In addition, it was reported that patients under the age of 20 years had a higher risk of developing LN metastasis.[23] The correlation coefficient between age and cervical LN involvement in our study was weakly negative (r = −0.154, P = 0.2), which indicates that cervical lymph involvement was more common in younger individuals and we are in agreement with other studies that recommended CLND in this age group.[23]
Regarding gender, despite claims to the contrary, studies conducted in the past revealed that men were more likely to develop cervical LN metastasis than women.[24,25] Comparatively, we found in our study that of the 23 patients with cervical LN metastasis, 17 (73.9%) were females and 6 (26.1%) were males.
Another independent predictor of LN metastasis is multifocal disease.[26] According to numerous studies, multifocal carcinomas make up between 20.3% and 33.5% of PTC.[24,27] In our study, 86 patients with PTC were diagnosed; of these, 59 patients (68.6%) had unifocal disease, and 27 patients (31.4%) had multifocal disease. Of the 27 patients with multifocal disease, 14 patients (51.9%) had cervical LN involvement. On the other side, of the 59 patients who had unifocal disease; 23 (38.9%) of them had cervical LN metastasis, which means that the disease is more common in patients with multifocal disease than unifocal disease.
Another prognostic factor is the size of the tumor. The American Thyroid Association published guidelines for the various DTC management options based on tumor size in 2015. For patients with T1a DTC, only unilateral lobectomy was advised; prophylactic central neck dissection was not advised for either T1 or T2 patients; and only patients with thyroid nodules > 4 cm complicated by extrathyroidal invasion were advised to undergo central neck dissection.[16] The correlation coefficient between the tumor size and cervical LN involvement, however, was only moderately strong in our study (r = 0.332, P < 0.05). Six (23%) out of the 23 patients who had cervical LN involvement had micropapillary carcinoma with tumor size <1 cm. Our results were consistent with other studies which reported a rate of metastatic LN as high as 12–64% in patients with PTC <1 cm.[26,28,29] Consequently, it was recommended that patients with micro PTC should not have prophylactic central neck dissection routinely. Still, the decision should be individualized based on multiple factors such as age, sex, location of the tumor, and other predictors.[23]
We have some limitations in our study. First, the sample size was relatively small. It may be attributed partially to including patients with unifocal PTC only and excluding patients with other subtypes or multifocal disease. Second, we collected our data retrospectively; however, we included the accurate data and any patient with missed or incomplete data were excluded from the study. Third, we did not consider other predictors such as tumor location or the patients’ presentation because our main aim was to compare the tumor size with the cervical LN involvement.
CONCLUSION
The decision regarding CLND should be selected on an individual basis as routine performance could increase the risk of permanent complications. Many factors could contribute to this decision including the patient’s age, gender, tumor size, location, and the presence or absence of involved lateral cervical LNs. Although the tumor size is an important predictor factor, the correlation between the size of the tumor and cervical LN involvement was relatively weak, which means that even small micro PTC could metastasize to the local LNs.
Ethical approval
Ethical approval was not required as the data included in our study was retrospectively collected, fully anonymised, and not including any biological samples, as per the University of Oxford Guides.
Declaration of patient consent
Patient’s consent not required as the figures are retrospectively collected, fully anonymised and does not include any patient’s details in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Familial nonmedullary thyroid carcinoma. Thyroid. 2013;23:1049-56.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment approach, surveillance, and outcome of well-differentiated thyroid cancer in childhood and adolescence. Thyroid. 2014;24:1121-6.
- [CrossRef] [PubMed] [Google Scholar]
- A snapshot of thyroid cancer. 2014. Available from: https://www.cancer.gov/researchandfunding/snapshots/thyroid [Last accessed on 2015 Jan 12]
- [Google Scholar]
- Clinically significant prognostic factors for differentiated thyroid carcinoma: A population-based, nested case-control study. Cancer. 2006;106:524-31.
- [CrossRef] [PubMed] [Google Scholar]
- Central cervical lymph node metastases in papillary thyroid cancer: A systematic review of imaging-guided and prophylactic removal of the central compartment. Clin Endocrinol (Oxf). 2012;76:131-6.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of prophylactic central neck lymph node dissection on early recurrence in papillary thyroid carcinoma. World J Surg. 2010;34:1187-91.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of preoperative ultrasonographic staging of the neck in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2007;133:1258-62.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: Comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid. 2008;18:411-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of RET/PTC rearrangement in benign and malignant thyroid nodules and its clinical application. Endocr J. 2011;58:31-8.
- [CrossRef] [PubMed] [Google Scholar]
- Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid. 2009;19:1153-8.
- [CrossRef] [PubMed] [Google Scholar]
- The incidence of central neck micrometastatic disease in patients with papillary thyroid cancer staged preoperatively and intraoperatively as N0. Surgery. 2011;150:1161-7.
- [CrossRef] [PubMed] [Google Scholar]
- Papillary thyroid carcinomas with cervical lymph node metastases can be stratified into clinically relevant prognostic categories using oncogenic BRAF, the number of nodal metastases, and extra-nodal extension. Thyroid. 2012;22:575-84.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation analysis on central lymph node metastasis in 276 patients with cN0 papillary thyroid carcinoma. Int J Clin Exp Pathol. 2013;6:510-5.
- [Google Scholar]
- A systematic review and meta-analysis comparing surgically-related complications between robotic-assisted thyroidectomy and conventional open thyroidectomy. Ann Surg Oncol. 2014;21:850-61.
- [CrossRef] [PubMed] [Google Scholar]
- A meta-analysis of the effect of prophylactic central compartment neck dissection on locoregional recurrence rates in patients with papillary thyroid cancer. Ann Surg Oncol. 2013;20:3477-83.
- [CrossRef] [PubMed] [Google Scholar]
- 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1-133.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical determinants of fluorodeoxyglucose positron emission tomography/computed tomography in differentiated thyroid cancer patients with elevated thyroglobulin and negative (131) iodine whole body scans after (131) iodine therapy. Malays J Med Sci. 2014;21:38-46.
- [Google Scholar]
- 18F-FDG PET/CT of papillary carcinoma in a lateral thyroglossal duct cyst. Clin Nucl Med. 2017;42:e371-4.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the diagnostic performances of ultrasonography, CT and fine needle aspiration cytology for the prediction of lymph node metastasis in patients with lymph node dissection of papillary thyroid carcinoma: A retrospective cohort study. Int J Surg. 2018;51:145-50.
- [CrossRef] [PubMed] [Google Scholar]
- Roles of ultrasonography and computed tomography in the surgical management of cervical lymph node metastases in papillary thyroid carcinoma. Eur J Surg Oncol. 2013;39:191-6.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factor analysis for central nodal metastasis in papillary thyroid carcinoma. Oncol Lett. 2015;9:103-7.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors of central lymph node metastasis of papillary thyroid carcinoma: A single-center retrospective analysis of 3273 cases. Medicine (Baltimore). 2017;96:e8365.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma: A study of 1066 patients. J Clin Endocrinol Metab. 2012;97:1250-7.
- [CrossRef] [PubMed] [Google Scholar]
- Pattern and predictive factors of regional lymph node metastasis in papillary thyroid carcinoma: A prospective study. Head Neck. 2013;35:40-5.
- [CrossRef] [PubMed] [Google Scholar]
- Central lymph node metastasis is an important prognostic factor in patients with papillary thyroid microcarcinoma. J Korean Med Sci. 2014;29:48-52.
- [CrossRef] [PubMed] [Google Scholar]
- Papillary thyroid microcarcinomas: A comparative study of the characteristics and risk factors at presentation in two cancer registries. J Clin Endocrinol Metab. 2013;98:1427-34.
- [CrossRef] [PubMed] [Google Scholar]
- Lateral lymph node metastasis in papillary thyroid carcinoma: Results of therapeutic lymph node dissection. Thyroid. 2009;19:241-6.
- [CrossRef] [PubMed] [Google Scholar]
- Central lymph node metastasis in papillary thyroid microcarcinoma can be stratified according to the number, the size of metastatic foci, and the presence of desmoplasia. Surgery. 2015;157:111-8.
- [CrossRef] [PubMed] [Google Scholar]






