Translate this page into:
Efficacy of screening cancer patients at hospital entrance for COVID-19 with a questionnaire and thermal scanning: An audit
*Corresponding author: Rajendra A. Badwe, Department of Surgical Oncology, Tata Memorial Centre, Homi Bhabha National Institute (HBNI), Maharashtra, India. badwera@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mishra GA, Goel NS, Gupta S, Laskar S, Tiloda AV, Bhagwat AA, et al. Efficacy of screening cancer patients at hospital entrance for COVID-19 with a questionnaire and thermal scanning: An audit. Indian J Med Sci 2022;74:10-4.
Abstract
Objectives:
Although commonly practiced, the accuracy, effectiveness, and safety of screening patients for COVID-19 at hospital entrances is not well documented.
Material and Methods:
We performed a retrospective analysis of single institution data involving screening patients for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at hospital entrances by trained health personnel, with thermal scanning and administration of a standard questionnaire eliciting risk factors and symptoms of COVID-19. SARS-CoV-2 positivity among patients screened positive and negative and among personnel involved in screening were estimated.
Results:
Between May 22, 2020, and July 4, 2020, a total of 20152 patients involving 54955 hospital visits were screened at hospital entrances of whom 668 (3.31%, 95% CI 3.07–3.57) were screened positive for suspected COVID-19 and 19484 (96.69%, 95% CI 96.44–96.93) were screened negative. Among patients screened positive, of the 638 patients with available records, 109 (17.08%, 95% CI 14.24–20.23) were confirmed to be SARS-CoV-2 positive by polymerase chain reaction test, 288 (45.14%, 95% CI 41.23–49.10) were negative, 71 (11.13%, 95% CI 8.79–13.83) were not tested after secondary assessment, and 170 (26.65%, 95% CI 23.25–30.26) patients declined the test. Among screen negative patients, 162 (0.83%, 95% CI 0.71–0.97) were SARS-CoV-2 positive. Of the 104 personnel involved in screening, 03 (2.88%, 95% CI 0.60–8.20) were confirmed to be SARS-CoV-2 positive during study period.
Conclusion:
Screening patients with a combination of thermal scanning and a standard questionnaire for COVID-19 has a high positive predictive value for detecting this infection with low risk of SARS-COV-2 transmission to the involved health personnel.
Keywords
Screening
COVID-19
Cancer
Pandemic
INTRODUCTION
The current COVID-19 pandemic has affected the entire health-care sector with unprecedented challenges and has led to disruption of healthcare and medical services for most other diseases as well. There have been reports of cancer patients not seeking or receiving adequate treatment because of fear of viral transmission during hospital visits and non-functionality of many institutions. Such situations not only increase the risk of direct mortality from the viral outbreak but also the indirect mortality from preventable and treatable conditions.[1] Further, all resources, including health-care personnel, are scarce in low- and middle-income countries and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among them further worsens the situation.
Screening individuals at entry points of various institutions, including hospitals have been commonly practiced since the beginning of the COVID-19 pandemic but it’s effectiveness in detecting SARS-CoV-2 infection remains unproven. Further, the risk of transmission to personnel involved in screening has also not been well documented.
Based on the low population-level COVID-19 mortality in India thus[2] far and the need for delivery of vital cancer care services, our institution, the largest tertiary care cancer center in India managing over 70,000 new cancer patients and 450,000 follow-up patients annually, decided to continue hospital operations since the beginning of the pandemic.[3] We also implemented screening of patients and caregivers for possible SARS-CoV-2 infection at hospital entrances since the beginning of the first lockdown in India on March 25, 2020. This activity was undertaken by trained paramedical health-care personnel. We analyzed, and report here, the effectiveness and safety of screening cancer patients and their accompanying persons at the entrances of our institution.
MATERIAL AND METHODS
With the spread of COVID-19 across Mumbai, several policies were implemented in our institution to mitigate the risk to staff, cancer patients and care givers and ensure effective hospital functioning. One of these was screening patients and accompanying persons for risk factors and symptoms suggestive of COVID-19 and undertaking their thermal screening before entry into the hospital. The screening was conducted by the trained paramedical health care personnel, including KEVATS (patient navigators) and other staff deputed from various departments of the hospital. The personnel involved in screening were trained to administer a COVID-19 questionnaire and thermal screening, with predefined objective criteria to identify individuals with suspected COVID-19. The trained screening staff also provided masks to patients and accompanying persons if required.
The questionnaire included, in addition to date and patient identifiers, the following items as questions: History of travel to or residence in COVID-19 high incidence area in the preceding 15 days, direct contact with a COVID-19 positive case in the preceding 15 days and symptoms of fever, sore throat, cough, or shortness of breath during past 5 days. The same questionnaire was also administered to person(s) accompanying the patient. Immediately after administration of questionnaire the temperature was recorded using hand-held infrared thermal scanners. A temperature recording of 99° Fahrenheit or above was considered as screen positive. An affirmative answer to any screening question or temperature above the threshold was categorized as being primary screen positive. The time taken to administer the questionnaire and thermal screening was measured by independent observers using a stop watch in a sample of screened patients.
Screening was undertaken round the clock and the staff deployed in shifts. The entry of patients was restricted to four entrances of the hospital. If a patient was found to be positive on primary screening, they were referred to the fever clinic established in the hospital. If accompanying persons were found to be positive, they were referred to a nearby government facility for testing. Others who were screened negative at the entrance were directed to access the respective hospital services.
In the fever clinic, a secondary screening was conducted by repeat history taking, recording temperature by clinical thermometer, and recording oxygen saturation. In all patients suspected to have high probability of having COVID-19 after secondary screening, laboratory specimens were collected by nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 testing by real-time reverse transcription–polymerase chain reaction (RT-PCR). Specimens were not collected for patients whose symptoms were more likely to be assigned to the primary disease condition or an ongoing treatment and such patients were referred to their treating physicians. Patients who tested negative for SARS-CoV-2 by RT-PCR were also directed to access their respective hospital services. All patients who tested positive for SARS-CoV-2 were admitted in isolation wards within the hospital premises and managed for COVID-19 and their primary cancer condition. All patients with inconclusive report were advised repeat testing after 24 h. Patients attending the various outpatient departments who were negative on primary screening at the entrance, but were assessed to have clinical features suggestive of COVID-19 on repeat evaluation by their respective physicians were also redirected to the fever clinic for SARS-CoV-2 testing.
The paramedical health-care personnel involved in the screening were trained to safeguard themselves from SARSCoV-2 transmission with appropriate use of personal protective equipment (PPE), social distancing, and hand hygiene. Each staff member was provided with a PPE kit before each shift that included a reusable cotton gown, one N-95 and 3-ply surgical mask each, one face shield, a pair of gloves, and hand sanitizers in a small dispenser. Their duty hours were restricted to 8 h in one shift with maximum of one shift per 24 h.
The results of SARS-CoV-2 testing were estimated as simple proportions with their 95% confidence intervals and descriptively reported. The flow chart of the methodology followed is shown in [Figure 1].
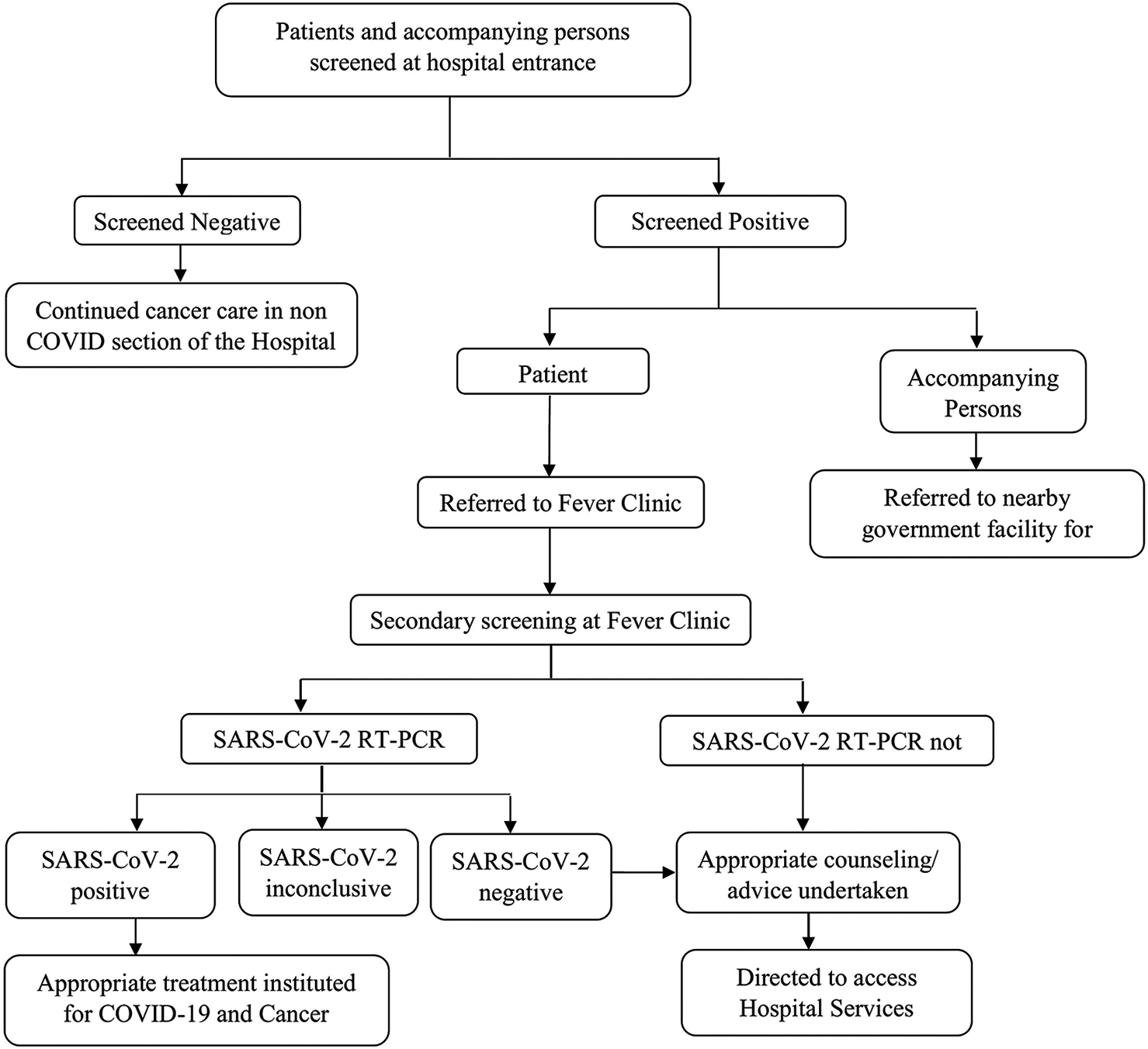
- CONSORT methodology.
RESULTS
Data of the 6 weeks period from May 22, 2020, to July 4, 2020, were considered for this analysis. The result of primary screening of patients is presented in [Figure 2]. A total of 54,955 patient visits, involving 20,152 patients were recorded at the four hospital entrances during this period. The times required for thermal screening and administration of questionnaire were measured by independent observers in 200 and 140 patients, respectively. The median (interquartile) times required for thermal screening and administration of screening questionnaire were 3 (3.00–4.00) s and 39.00 (30.00–45.00) s, respectively [Figure 2].
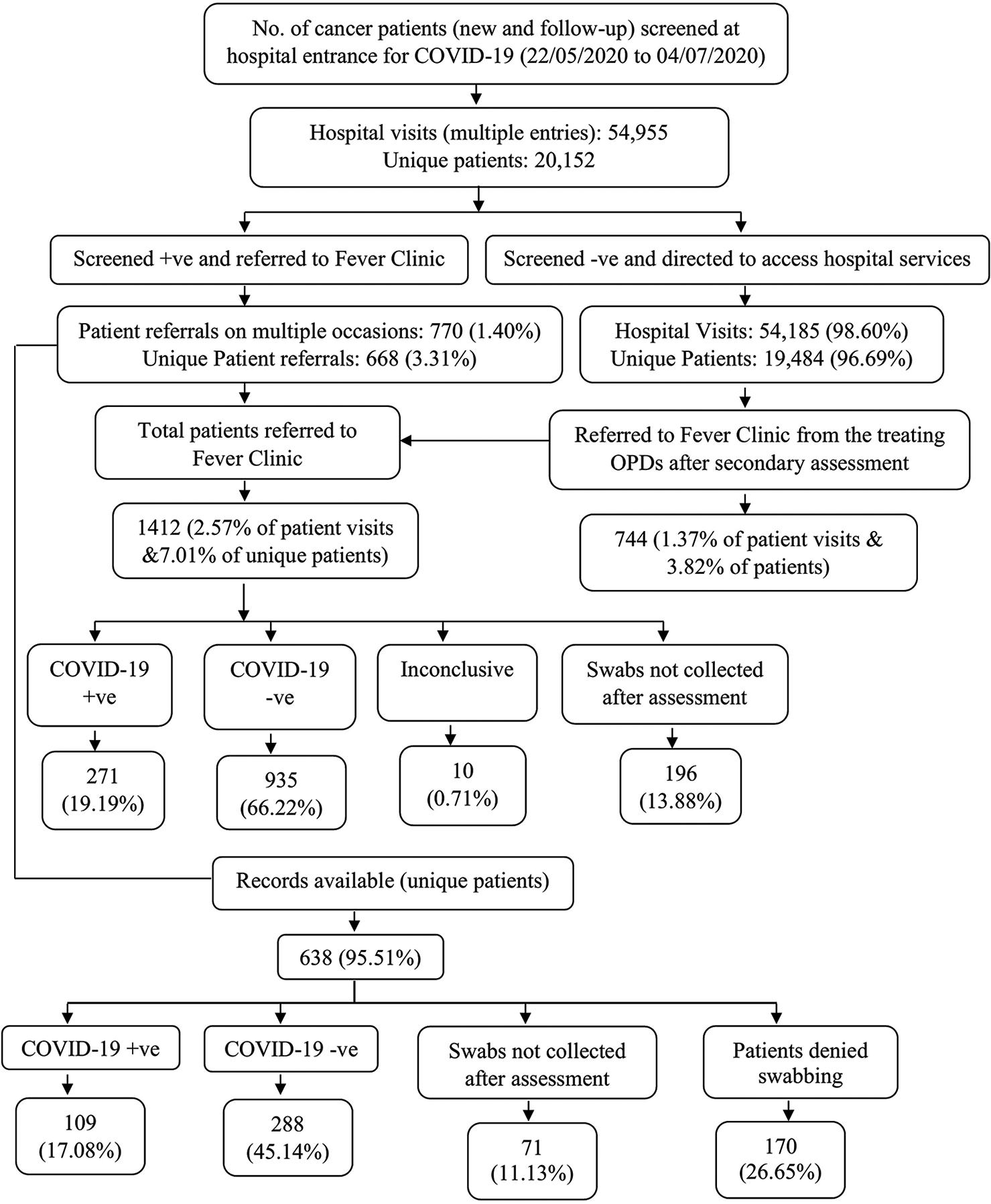
- Screening of cancer patients for COVID-19.
On primary screening, 770 patient visits (1.40%, [95% CI: 1.30–1.50]) involving 668 (3.31%, [95% CI: 3.07–3.57]) patients were positive for suspected SARSCoV-2 infection and referred to the fever clinic while 54,185 hospital visits (98.60%, [95% CI: 98.50–98.70]) involving 19,484 (96.69%, [95% CI: 96.44–96.93]) patients were screen negative. Of the screen negative results, 744 (3.82%, [95% CI: 3.55–4.10]) patients were considered by their treating physicians to have probable COVID-19 and referred for SARS-CoV-2 testing. Among the latter 162 (0.83%, [95% CI: 0.71–0.97]) patients were found to have SARS-CoV-2 positive results constituting the false negative rate of primary screening.
Of the 668 primary screening positive patients, records were available for 638 (95.51%) patients and were not available for the remaining 30 patients. Among these 638 patients, 109 (17.08%, [95% CI: 14.24–20.23]) were positive for SARS-CoV-2, 288 (45.14%, [95% CI: 41.23–49.10]) were negative for SARSCoV-2, and 241 (37.77%, [95% CI: 34.00–41.66]) were not tested. Of those not tested, secondary screening did not suggest possible COVID-19 in 71 (11.13%, [95% CI: 8.79–13.83]) patients, while 170 (26.65%, [95% CI: 23.25–30.26]) patients declined SARSCoV-2 test. Considering only the 397 patients who were tested, SARS-CoV-2 positivity was 27.46% (95% CI: 23.12–32.13).
All 271 cancer patients who were diagnosed to have COVID-19 during the study period were admitted in the hospital and provided appropriate medical care. One hundred and four paramedical staff were involved in primary screening and they performed this activity for mean and median durations of 87.00 (± 58.64) h and 72.00 (40.00– 112.00) h, respectively, during the 6-week study period. Of them, 03 (2.88%, [95% CI: 0.60–8.20]) staff tested positive for SARS-CoV-2 during this period.
DISCUSSION
The results of our study suggest that screening patients and their caregivers at hospital entrance with a combination of thermal and questionnaire-based screening had a high positive predictive value and low false negative rate for SARS-COV-2 infection. This screening could be rapidly accomplished by trained paramedical staff members who had a low risk of transmission of this infection to themselves. Our analysis is one of the few which have quantified and established the effectiveness and safety of this procedure which is widely practiced by many institutions all over the world. The potential benefits of this screening procedure include prevention of viral transmission to other cancer patients and health-care personnel, as well as early monitoring and treatment of cancer patients with SARS-CoV-2 infection. The latter is particularly important because cancer patients have been shown to be at high risk of COVID-19 related complications in several studies.[4-6]
A study from China[7] which analyzed the results of thermal screening of patients entering the hospital, reported positive predictive values of 1.40% and 3.31% for analyzed hospital visits and patients, respectively, which are much lower than the positive predictive value of 17.08% for primary screen positive patients in our study. This suggests that thermal screening by itself is insufficient as a screening tool for detection of SARS-CoV-2 infection. It also suggests that administration of a simple questionnaire comprising simple and rapidly administered questions related to COVID-19 epidemiology and symptoms can substantially increase the specificity of screening. Of note, the false negative rate of our screening methodology was low (0.83%) which also suggests that it has relatively high sensitivity for its intended purpose.
Because of the widespread transmission of SARS-CoV-2 in the community, it has become increasingly complex and difficult to protect vulnerable populations such as cancer patients from this infection. Other strategies that have been employed include mandatory testing of all cancer patients before definitive treatments[8] or at regular intervals. However, this strategy is resource intensive and likely results in straining the already stretched laboratory services. In contrast our screening strategy is widely implementable and feasible on realistic timescales. This screening protocol helped our institution to continue providing healthcare to cancer patients who could have otherwise experienced adverse outcomes due to delayed diagnosis or treatment.[9]
Our results also suggest that the screening procedure is relatively safe for personnel who undertake it, as shown by the low rate of SARS-CoV-2 infection among them. This likely resulted from the provision of appropriate protective equipment, meticulous training, and adherence to operating procedures among these personnel. The strength of our study is the large sample size of screened patients which indicates the feasibility of our screening procedure as well as the precision of our results. The limitations of our analysis include some data that were missing and a proportion of screen positive patients who declined SARS-CoV-2 testing. We could not analyze the contribution of screening in caregivers of patients because they were not tested at our institution. Our data capture also did not allow us to analyze the relative contributions of thermal screening and the individual items of the questionnaire to the results. As the pandemic evolves, we and others will refine the screening strategies including the content of the questionnaire to improve its accuracy.
CONCLUSION
A simple and rapidly administered screening procedure involving thermal and questionnaire-based screening was accurate and effective in screening for possible SARS-CoV-2 infection among cancer patients visiting a large tertiary care cancer centre. As we return to pre-COVID levels of activity, this could be an easily implementable screening procedure to ensure safety of workforce and resumption of normal functionality in medical and non-medical organizations.
Declaration of patient consent
Patients consent was not required as patients identity was not disclosed or compromised. The approval for waiver of consent was obtained from Institutional Ethics Committee.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Maintaining Essential Health Services: Operational Guidance for the COVID-19 Context Interim Guidance 1 June 2020 Geneva: World Health Organization; 2020.
- [Google Scholar]
- 2020. Available from: https://www.worldometers.info/coronavirus/country/india [Last accessed on 2020 Aug 04]
- [Google Scholar]
- Cancer management in India during COVID-19. N Engl J Med. 2020;382:e61.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020;21:335-7.
- [CrossRef] [Google Scholar]
- Cancer and COVID-19: Unmasking their ties. Cancer Treat Rev. 2020;88:102041.
- [CrossRef] [PubMed] [Google Scholar]
- Application and effects of fever screening system in the prevention of nosocomial infection in the only designated hospital of Coronavirus disease 2019 (COVID-19) in Shenzhen, China. Infect Control Hosp Epidemiol. 2020;41:978-81.
- [CrossRef] [PubMed] [Google Scholar]
- SARS-CoV-2 testing in patients with cancer treated at a tertiary care hospital during the COVID-19 pandemic. J Clin Oncol. 2020;38:3547-54.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of elective major cancer surgery during COVID 19 at Tata Memorial Centre: Implications for cancer care policy. Ann Surg. 2020;272:e249-52.
- [CrossRef] [PubMed] [Google Scholar]






