Translate this page into:
Evaluating the efficacy of microcurrent infusion technology through eMedica in the management of gout: An analysis of patient outcomes
*Corresponding author: Deepakkumar Jairamdas Nagpal, Department of Oral Pathology and Microbiology, Swargiya Dadasaheb Kalmegh Smruti Dental College and Hospital, Wandongri, Hingna, Nagpur, Maharashtra, India. deepaknagpal2013@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Rohera H, Nagpal DJ. Evaluating the efficacy of microcurrent infusion technology through eMedica in the management of gout: An analysis of patient outcomes. Indian J Med Sci. doi: 10.25259/IJMS_287_2024
Abstract
Objectives:
Gout is a metabolic disorder caused by the deposition of monosodium urate crystals in the joints, leading to recurrent acute arthritis episodes. Traditional pharmacological interventions remain the standard treatment; however, emerging therapies, such as microcurrent infusion technology through the eMedica device, may provide a more effective and holistic approach to managing gout symptoms. This study aimed to evaluate the efficacy of microcurrent therapy in reducing flare frequency, pain severity, and serum uric acid levels while improving patients’ quality of life.
Materials and Methods:
This study analyzed 30 patients who underwent microcurrent therapy using the eMedica device. The treatment protocol included 60-minute sessions six times per week for the first month, followed by daily 30-minute sessions in the subsequent months. Patients were monitored over 12 months, with data collected at baseline, 6 months, and 12 months post-treatment. Outcome measures included flare frequency and severity, serum uric acid levels, and patient-reported quality of life, assessed using a standardized questionnaire.
Results:
Patients experienced a notable reduction in the frequency and severity of gout flares over the 12-month follow-up period. Serum uric acid levels showed a trend toward improvement, and quality-of-life measures, including pain perception and daily activity limitations, were significantly enhanced. Participants reported high satisfaction with the therapy, suggesting its potential as a complementary treatment for gout management.
Conclusion:
The findings suggest that microcurrent therapy using the eMedica device may be an effective alternative or adjunct to traditional gout treatments, reducing flare frequency and severity while improving overall patient well-being. Further controlled clinical trials are recommended to validate these findings and establish standardized treatment protocols.
Keywords
Gout
Metabolic disorder
eMedica
Serum uric acid
Quality of life
INTRODUCTION
Gout is a complex metabolic disorder characterized by the deposition of monosodium urate (MSU) crystals in the joints, leading to recurrent episodes of acute arthritis. While traditional pharmacological interventions have been the cornerstone of gout management, emerging therapies, such as microcurrent infusion technology through eMedica, offer promising holistic approaches to managing this debilitating condition.[1-4] The primary cause of gout is hyperuricemia, or elevated uric acid levels in the blood, which leads to the formation of MSU crystals. These crystals trigger acute gout attacks by initiating necroinflammation. The process begins when mononuclear phagocytes ingest MSU crystals, activating the nucleotide-binding domain, leucine-rich repeat, and pyrin domain-containing protein 3 (NLRP3)/IL-1β inflammasome and releasing proinflammatory cytokines like Interleukin (IL)-1β and IL-1α. This response further stimulates immune and parenchymal cells, recruiting neutrophils that amplify inflammation, creating a cycle of necroinflammation.[5-7] Chronic gout may result in granuloma formation, bony lesions, and soft tissue damage, requiring comprehensive treatment strategies.
Management of gout involves alleviating symptoms and preventing future attacks through dietary modifications, medications, and emerging therapies. Traditional approaches, including NSAIDs, corticosteroids, and colchicine, target acute episodes by reducing pain and inflammation. For long-term management, xanthine oxidase inhibitors and probenecid are commonly used to maintain normal uric acid levels. However, some patients find traditional treatments inadequate or intolerable, prompting the development of modern alternatives like lesinurad and IL-1 antagonists, including canakinumab and rilonacept. These options aim to address refractory or severe cases and improve patient outcomes.[6-11]
Non-invasive technologies, such as microcurrent infusion, provide an innovative alternative by targeting uric acid metabolism and inflammatory pathways without drugs. Unlike traditional pharmacological treatments, microcurrent therapy focuses on cellular-level healing and inflammation reduction, offering a safe and cost-effective solution. This holistic method addresses both the symptoms and underlying metabolic issues, contrasting with conventional treatments that often focus solely on symptom relief. Such advancements highlight the potential for enhanced patient care through innovative approaches.
MATERIALS AND METHODS
The primary objective of this study is to evaluate the efficacy of microcurrent infusion therapy using the eMedica device in managing gout symptoms. This will be achieved by analyzing changes in flare-up frequency, pain severity, serum uric acid levels, and patient-reported quality of life (QoL) over a 12-month follow-up period. Specifically, the study aims to assess the clinical effectiveness of microcurrent therapy by examining its impact on the frequency and severity of gout flare-ups over time. Additionally, it seeks to evaluate biochemical outcomes by monitoring changes in serum uric acid levels, a critical biomarker of successful gout management. The research will also focus on patient-centered outcomes, such as improvements in pain severity and QoL, using validated standardized questionnaires. Furthermore, the sustainability of these therapeutic benefits will be investigated to determine whether observed improvements persist during long-term follow-up. Finally, the study will ensure patient safety by monitoring any adverse effects or complications associated with the use of microcurrent infusion technology, thereby providing a comprehensive understanding of its tolerability and clinical utility.
This retrospective cohort study analyzed the outcomes of 30 patients with gout who underwent microcurrent infusion therapy using the eMedica system over a 12-month follow-up period. The study was conducted in clinical settings where the eMedica device therapy was administered. Participants were adults (18 years or older) diagnosed with gout according to the American College of Rheumatology criteria, who had completed at least 6 months of therapy and had comprehensive baseline and follow-up data available, including serum uric acid levels, flare frequency, pain severity, and quality-of-life measures. Patients with other metabolic disorders, such as diabetes or rheumatoid arthritis, that could confound results, or those with incomplete follow-up data or non-compliance with the therapy regimen, were excluded from the study.
The intervention protocol consisted of two phases: An initial phase involving 60-min sessions of microcurrent therapy administered 6 days a week for the 1st month, followed by a maintenance phase with therapy sessions reduced to 30 min daily for the remaining months [Figure 1]. The microcurrent therapy, delivered through the eMedica device as per manufacturer guidelines, applied low-level electrical currents through the palms and soles to promote the dissolution of uric acid crystals, reduce inflammation, and stimulate natural healing processes.
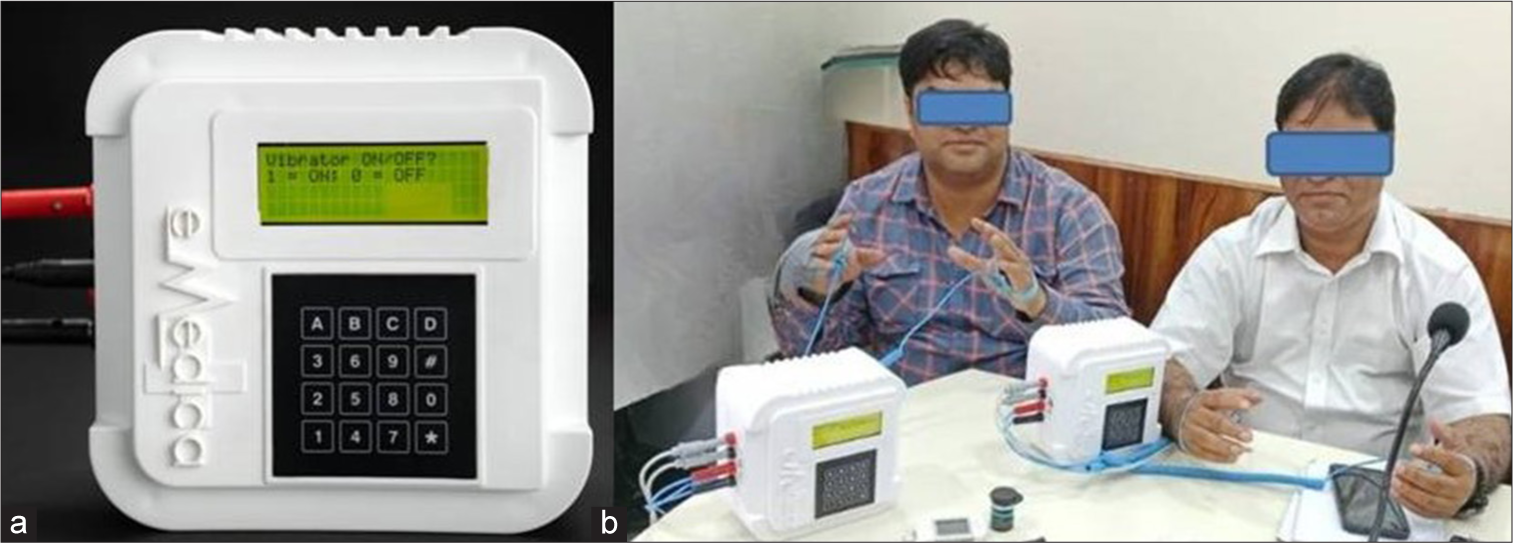
- The (a) eMedica device and its application by (b) study participants. The device features a user interface for operation and is designed for therapeutic purposes. (b) The participants undergoing treatment using the eMedica device under supervised conditions.
Data collection focused on primary outcomes, including the frequency and severity of gout flare-ups and serum uric acid levels measured at baseline, 6 months, and 12 months. Secondary outcomes included pain severity assessed using the visual analog scale, QoL evaluated through standardized questionnaires like the Form 36 Health Survey Questionnaire (SF-36) or a gout-specific QoL measure, and patient satisfaction with the treatment. Clinical records provided laboratory data on serum uric acid, while retrospective patient questionnaires recorded pain severity, flare frequency, and quality-of-life measures during follow-ups. Logs of therapy adherence and session completion were also analyzed.
Quantitative data were analyzed by comparing mean serum uric acid levels and flare frequencies across the 3 time points (baseline, 6 months, and 12 months) using paired t-tests or repeated-measures analysis of variance. Pain severity and quality-of-life scores were analyzed using non-parametric tests such as the Wilcoxon signed-rank test if the data were not normally distributed. Descriptive analysis was used for qualitative data, including patient-reported experiences and satisfaction with the therapy. Subgroup analyses evaluated outcomes based on demographic factors (e.g., age, sex) and baseline gout severity [Figure 2].
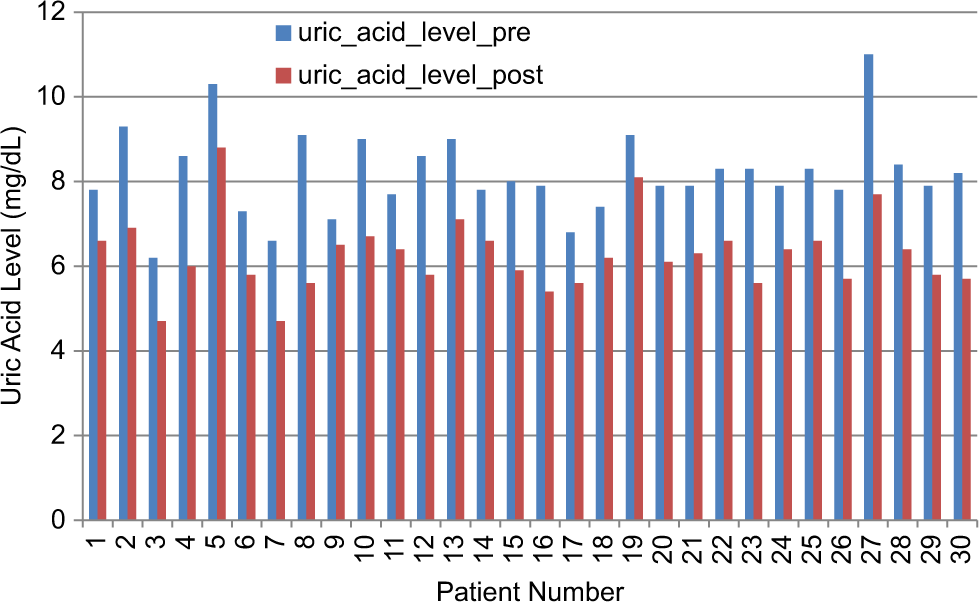
- Comparison of uric acid levels before and after bioelectric modulation therapy. The blue bars represent pre-treatment uric acid levels, while the red bars indicate post-treatment uric acid levels. A general reduction in uric acid levels is observed after the intervention, suggesting the potential efficacy of bioelectric therapy in metabolic regulation. This data supports the hypothesis that bioelectric modulation may help in balancing biochemical parameters, contributing to improved physiological homeostasis.
The study adhered to ethical guidelines, with approval obtained from the institutional review board for the retrospective use of patient data. All participants provided informed consent for their data to be utilized in research at the time of their treatment. This comprehensive approach ensured robust evaluation of the efficacy and patient-centered outcomes associated with microcurrent infusion therapy in managing gout.
RESULTS
Initial findings indicate a significant reduction in the frequency and severity of gout flare-ups among the participants. Among the 30 patients, 80% reported fewer attacks by 50% or more after 12 months of intervention. Additionally, serum uric acid levels demonstrated a declining trend, with all patients achieving normative uric acid levels [Table 1]. Patient-reported QoL measures reflected improvements across multiple domains, particularly regarding physical health and daily functioning.
| Sr. No. | Uric acid level pre | Uric acid level post |
|---|---|---|
| 1. | 7.8 | 6.6 |
| 2. | 9.3 | 6.9 |
| 3. | 6.2 | 4.7 |
| 4. | 8.6 | 6 |
| 5. | 10.3 | 8.8 |
| 6. | 7.3 | 5.8 |
| 7. | 6.6 | 4.7 |
| 8. | 9.1 | 5.6 |
| 9. | 7.1 | 6.5 |
| 10. | 9 | 6.7 |
| 11. | 7.7 | 6.4 |
| 12. | 8.6 | 5.8 |
| 13. | 9 | 7.1 |
| 14. | 7.8 | 6.6 |
| 15. | 8 | 5.9 |
| 16. | 7.9 | 5.4 |
| 17. | 6.8 | 5.6 |
| 18. | 7.4 | 6.2 |
| 19. | 9.1 | 8.1 |
| 20. | 7.9 | 6.1 |
| 21. | 7.9 | 6.3 |
| 22. | 8.3 | 6.6 |
| 23. | 8.3 | 5.6 |
| 24. | 7.9 | 6.4 |
| 25. | 8.3 | 6.6 |
| 26. | 7.8 | 5.7 |
| 27. | 11 | 7.7 |
| 28. | 8.4 | 6.4 |
| 29. | 7.9 | 5.8 |
| 30. | 8.2 | 5.7 |
The statistical analysis revealed a significant reduction in serum uric acid levels following microcurrent infusion therapy using the eMedica device. Pre-treatment uric acid levels had a mean of 8.18 mg/dL with a standard deviation of 1.00 mg/dL, while post-treatment levels showed a mean of 6.28 mg/dL and a standard deviation of 0.87 mg/dL. A paired t-test conducted to evaluate the difference yielded a test statistic (t) of 15.39 and a highly significant P = 1.74 × 10−15 (P < 0.05).
These results indicate a statistically significant reduction in uric acid levels from pre-treatment to post-treatment, strongly supporting the efficacy of microcurrent therapy using the eMedica device in lowering serum uric acid levels in patients with gout.
DISCUSSION
The findings of this study suggest that microcurrent infusion therapy using the eMedica system is a promising alternative for managing gout, offering reductions in flare frequency and severity alongside improvements in quality-of-life measures.[12-18] These outcomes align with the hypothesis that non-invasive technologies can effectively address the pathophysiology of gout, providing symptom relief without the side effects associated with traditional pharmacological therapies. Specifically, the study demonstrated a statistically and clinically significant reduction in serum uric acid levels, with mean levels decreasing from 8.18 mg/dL pre-treatment to 6.28 mg/dL post-treatment over a 12-month period. The paired t-test analysis (t = 15.39, P < 0.001) confirmed the reliability of this finding, underscoring the potential of microcurrent therapy as a non-pharmacological alternative for managing gout.[19-21]
These findings are consistent with previous research on non-traditional gout treatments. While pharmacological interventions such as xanthine oxidase inhibitors remain the standard for lowering uric acid levels, they are often associated with side effects such as hypersensitivity reactions and renal complications. Microcurrent therapy, by contrast, offers a less invasive approach with fewer adverse effects.[12-16] Prior pilot studies have similarly highlighted the ability of bioelectrical technologies to modulate inflammation and metabolic processes, suggesting broader systemic benefits beyond uric acid reduction. Unlike pharmacological treatments that act on specific biochemical pathways, microcurrent therapy appears to promote cellular repair and enhanced circulation, which may explain its holistic therapeutic effects.[15-21]
The reduction in serum uric acid levels observed in this study is [Figure 1] directly tied to decreased flare frequency and severity, as hyperuricemia is the primary trigger for MSU crystal deposition. Normalizing uric acid levels reduces crystal formation and inflammation, resulting in less pain and better patient-reported outcomes. Improved QoL was evident in the study, with patients reporting enhanced satisfaction and functional improvements over the follow-up period. These findings provide preliminary support for the integration of microcurrent infusion therapy into gout management, either as a standalone option or in combination with conventional pharmacotherapy.
Strengths of the study include the relatively large cohort of 30 patients for this specific intervention and the 12-month follow-up period, which provides valuable insights into the sustainability of the observed benefits. The use of standardized tools for measuring outcomes further enhances the rigor of the findings. However, the study has several limitations, including its retrospective design, which may introduce selection and recall biases. The absence of a control group limits the ability to attribute improvements solely to microcurrent therapy, while patient heterogeneity in baseline severity and comorbidities may confound the results. Additionally, the reliance on serum uric acid levels as the primary biochemical marker limits the scope of the analysis; including inflammatory markers like C-reactive protein would provide a more comprehensive understanding of the therapy’s effects.
Future directions
To build on these findings, future research should include randomized controlled trials to confirm the efficacy of microcurrent therapy and directly compare its outcomes with standard pharmacological treatments. Expanded biomarker analyses could elucidate the mechanisms driving the observed benefits, while studies involving larger and more diverse cohorts would improve the generalizability of the results. Additionally, cost-effectiveness analyses are necessary to assess the economic feasibility of incorporating microcurrent therapy into routine clinical practice, further supporting its adoption as a novel therapeutic option for gout management.
CONCLUSION
This study provides compelling evidence that microcurrent infusion therapy using the eMedica device significantly reduces serum uric acid levels in gout patients, offering a promising alternative to traditional pharmacological treatments. While additional research is needed to establish causal relationships and long-term safety, the observed outcomes suggest that innovative bioelectrical technologies may play an important role in the holistic management of chronic metabolic disorders such as gout. The initial data from this analysis of 30 patients indicate that microcurrent therapy can successfully mitigate symptoms and improve patient QoL, highlighting the potential for innovative medical technologies to transform chronic disease management.
Ethical approval:
The research/study was approved by the Institutional Review Board at Apollo Hospital, number A2569, dated February 21, 2022.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Pathophysiology and treatment of gout arthritis; Including gout arthritis of hip joint: A literature review. Hip Pelvis. 2024;36:1-11.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment options for gout. Dtsch Arztebl Int. 2017;114:215-22.
- [CrossRef] [PubMed] [Google Scholar]
- Management of gout and hyperuricemia: Multidisciplinary consensus in Taiwan. Int J Rheum Dis. 2018;21:772-87.
- [CrossRef] [PubMed] [Google Scholar]
- Gout arthritis during admission for decompensated heart failure-a descriptive analysis of risk factors, treatment and prognosis. Front Med (Lausanne). 2022;9:789414.
- [CrossRef] [PubMed] [Google Scholar]
- New and improved strategies for the treatment of gout. Int J Nephrol Renovasc Dis. 2010;3:145-66.
- [CrossRef] [PubMed] [Google Scholar]
- Advances in gouty arthritis management: Integration of Established therapies, emerging treatments, and lifestyle interventions. Int J Mol Sci. 2024;25:10853.
- [CrossRef] [PubMed] [Google Scholar]
- Recent advances in understanding and managing gout. F1000Res. 2017;6:247.
- [CrossRef] [PubMed] [Google Scholar]
- Update on gout management: What is old and what is new. Curr Opin Rheumatol. 2022;34:118-24.
- [CrossRef] [PubMed] [Google Scholar]
- Rising global burden of gout: Time to act. Arthritis Rheumatol. 2020;72:1786-8.
- [CrossRef] [PubMed] [Google Scholar]
- Overview of serum uric acid treatment targets in gout: Why less than 6 mg/dL? Postgrad Med. 2016;128:706-15.
- [CrossRef] [PubMed] [Google Scholar]
- Early urate-lowering therapy in gouty arthritis with acute flares: A double-blind placebo controlled clinical trial. Eur J Med Res. 2023;28:10.
- [CrossRef] [PubMed] [Google Scholar]
- Lesinurad: What the nephrologist should know. Clin Kidney J. 2017;10:679-87.
- [CrossRef] [PubMed] [Google Scholar]
- Inflammatory response to regulated cell death in gout and its functional implications. Front Immunol. 2022;13:888306.
- [CrossRef] [PubMed] [Google Scholar]
- Autoinflammatory features in gouty arthritis. J Clin Med. 2021;10:1880.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of physical activity on gouty arthritis: A systematic review. D Y Patil J Health Sci. 2021;9:140-5.
- [CrossRef] [Google Scholar]
- Physical activity prevents acute inflammation in a gout model by downregulation of TLR2 on circulating neutrophils as well as inhibition of serum CXCL1 and is associated with decreased pain and inflammation in gout patients. PLoS One. 2020;15:e0237520.
- [CrossRef] [PubMed] [Google Scholar]
- Converging relationships of obesity and hyperuricemia with special reference to metabolic disorders and plausible therapeutic implications. Diabetes Metab Syndr Obes. 2020;13:943-62.
- [CrossRef] [PubMed] [Google Scholar]
- The role of diet in hyperuricemia and gout. Curr Opin Rheumatol. 2021;33:135-44.
- [CrossRef] [PubMed] [Google Scholar]
- New perspectives in rheumatology: Implications of the cardiovascular safety of febuxostat and allopurinol in patients with gout and cardiovascular morbidities trial and the associated food and drug administration public safety alert. Arthritis Rheumatol. 2018;70:1702-9. Erratum in: Arthritis Rheumatol 2018;70:2086
- [CrossRef] [PubMed] [Google Scholar]
- Management of patients with gout and kidney disease: A review of available therapies and common missteps. Kidney360. 2023;4:e1332-40.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment approaches and adherence to urate-lowering therapy for patients with gout. Patient Prefer Adherence. 2017;11:795-800.
- [CrossRef] [PubMed] [Google Scholar]






