Translate this page into:
Hydroxychloroquine as prophylaxis when treating patients with coronavirus disease 2019 – Perception of frontline health care workers in South India
*Corresponding author: Karthik Mani, Department of Occupational Therapy, University of Texas Medical Branch, Galveston, Texas, United States. kmani@votc.co.in
-
Received: ,
Accepted: ,
How to cite this article: Mani K, Velan M. Hydroxychloroquine as prophylaxis when treating patients with coronavirus disease 2019 – Perception of frontline health care workers in South India. Indian J Med Sci 2021;73(2):141-6.
Abstract
Objectives:
In March 2020, the Indian Council of Medical Research (ICMR) recommended the use of hydroxychloroquine (HCQ) for prophylaxis in asymptomatic health care workers who care for suspected or confirmed patients and household contacts of confirmed patients with coronavirus disease 2019 (COVID-19). This recommendation was received by health-care practitioners with mixed opinions. The objectives of the study were to explore the views of frontline health-care practitioners (physicians, nurses, and physician assistants) in South India related to the ICMR recommendation of HCQ prophylaxis.
Material and Methods:
The survey research design was used to conduct this study. A ten-item electronic survey was developed based on the research question. The survey link was shared with frontline health-care practitioners in South India through email and WhatsApp messages identified through convenience sampling. Furthermore, the recipients were requested to forward the link to other frontline health-care professionals in their network (snowball sampling). Data were collected from April 16, 2020, to May 7, 2020.
Results:
The number of responses received was 132. Of 80 respondents who treated or anticipate treating patients with COVID-19, only 29 respondents reported that they complied with the ICMR’s HCQ chemoprophylaxis recommendation. Participants expressed concerns about the side effects and lack of conclusive evidence.
Conclusion:
Frontline health care workers in South India have mixed opinions with regard to the safety of HCQ prophylaxis. To promote the acceptance of and successful implementation of prophylaxis recommendations, it is essential that health authorities consider research evidence and seek stakeholder input before finalizing recommendations.
Keywords
Drug-related side effects and adverse reactions
Health personnel
Physicians
Prevention
Surveys and questionnaires
INTRODUCTION
India is second in the list of countries with most severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 aka coronavirus disease 2019 [COVID-19]) at the time of this writing.[1] Health care workers treating patients with COVID-19 are at higher risk of contracting the SARS-CoV-2 infection. In March 2020, the Indian Council of Medical Research (ICMR) published a bulletin in which it recommended the use of hydroxychloroquine (HCQ) (400 mg twice a day on day 1 and 400 mg once weekly for 7 weeks thereafter for health workers and 400 mg twice a day on day 1 and 400 mg once weekly for 3 weeks for household contacts) as a chemoprophylaxis for asymptomatic health care workers exposed to patients with COVID-19 and asymptomatic household contacts of laboratory-confirmed cases.[2] However, stakeholders raised concerns about the efficacy of chloroquine and HCQ in preventing COVID-19, as most of the available evidence is inconclusive.
A recent randomized, double-blind, placebo-controlled trial conducted in the United States and Canada revealed that HCQ did not prevent COVID-19 in health care workers exposed to COVID-19.[3] However, Cohen questioned the findings of this study by critically reviewing it and stated that the results of the trial were more provocative than definitive and the efficacy of HCQ as a chemoprophylaxis against COVID-19 is yet to be determined.[4] An early terminated randomized controlled trial (RCT) revealed that the use of HCQ did not prevent SARS-CoV-2 infection in health care workers.[5] Similar findings were also reported by Rajasingham et al. through a randomized trial involving health care workers in emergency departments, intensive care unit (ICU), COVID-19 hospital wards, and first responders.[6]
Literature is also equivocal on the efficacy of HCQ in reducing viral load and mortality in patients with COVID-19. In a large sample observational study conducted in Belgium, the authors found that the low dose (400 mg twice on day 1, followed by 200 mg twice a day from days 2 to 5) HCQ therapy reduced the in-hospital mortality rate by almost 30% when compared to patients treated with supportive care.[7] A retrospective cohort study conducted in New York also revealed that the HCQ use was associated with reduced in-hospital mortality.[8] A multicenter study conducted in Michigan also supported the use of HCQ alone or in combination with Azithromycin to reduce COVID-19-associated mortality.[9] Through a retrospective study in France, Lagier et al. found that early diagnosis, early isolation, and early treatment of COVID-19 patients with HCQ-azithromycin (200 mg of oral HCQ, 3 times daily for 10 days and 500 mg of oral AZ on day 1 followed by 250 mg daily for the next 4 days) therapy for at least 3 days lead to significantly better clinical outcome and reduced viral loads.[10]
Contrary to the above findings, the Recovery Collaborative Group, through an open-label RCT, reported that there was no difference in 28-day in-hospital mortality rate among patients with COVID-19 due to the use of HCQ.[11] In July 2020, the World Health Organization, based on the recommendation from its Solidarity Trail International Steering Committee, announced that it discontinues the HCQ arm of the interim trial as HCQ produced little or no reduction in in-hospital mortality of patients with COVID-19.[12] Cavalcanti et al., based on a multicenter study in Brazil, reported that the use of HCQ in patients with mild-to-moderate COVID-19 did not improve clinical status at 15 days when compared to standard care.[13]
Campbell argued that the negative findings must be approached with caution as the dosage of HCQ provided to study participants was high, relative to the recommended dosage for adults per British National Formula, in those studies that reported no or limited benefit of HCQ for prevention and treatment of COVID-19.[14]
These mixed findings and opinions of public health experts and other stakeholders created a sense of ambiguity among health-care professionals in India. This may have affected their compliance with the ICMRs recommendation. The aim of this study was to explore the perception of frontline health care workers (physicians, nurses, and physician assistants) in South India about ICMRs recommendation and related compliance.
MATERIAL AND METHODS
This study was conducted adhering to the principles of Declaration of Helsinki guidelines. The guidelines were reviewed before the survey being sent to the participants. In addition, the survey invitation that was sent to the participants clearly identified that participation in the survey was “strictly voluntary and the responses will be kept anonymous”.
The authors developed the survey by reviewing the literature and following survey writing guidelines. The survey tool consisted of 10 items. The first few items gathered information on participants’ demographics (gender, state, practice setting, unit/ward, and specialty). Subsequent items asked whether they were treating/anticipate treating patients with COVID-19, whether they were complaint with ICMRs recommendation on HCQ chemoprophylaxis, reason for non-compliance with the prophylaxis, and their view on safety of HCQ prophylaxis. The final open-ended item asked participants to express any other related thoughts.
The survey tool was reviewed by a few health-care practitioners in Tamil Nadu for face validity. All reviewers agreed that the survey tool was easy to understand. No changes were made to the original survey tool.
Study design
The study’s targeted population was frontline health-care professionals in South India. The participants were identified through convenience and snowball sampling. The authors shared the survey link through email and text messages to frontline health care workers in their network. Further, the survey invitation requested the participants to share the survey link with other suitable individuals in their network (snowball sampling).
The survey was conducted through SurveyMonkey™.[15] In April 2020, the survey link was shared with all identified participants through an email or text message by the authors. The survey link identified May 7, 2020, as the response deadline. A few reminder text messages were sent during the open survey period.[16] At the end of survey response period, all data were exported and tabulated for statistical analysis.
Statistical analysis
Descriptive statistics were used to summarize and report the responses received. Data are reported as percentages and aggregate numbers to protect the identity of respondents. Chi-square test was performed to determine the association between the variables as needed. The data were clustered logically when the frequency values were low. For instance, due to low number of responses, all states except Tamil Nadu were grouped as “other states,” “strongly agree,” and “agree” responses were grouped; “strongly disagree” and “disagree” responses were combined; etc. When the data were aggregated for statistical analyses, neutral responses were eliminated. The responses to the open-ended question were analyzed for themes.
RESULTS
By the response deadline, 132 responses were received. Of 132 respondents, 52% (n = 68) were male and 48% (n = 564) were female; 80% (n = 105) of respondents identified their geographical location (practice) as Tamil Nadu, 17% (n = 23) as Karnataka, and 2% (n = 2) as Telangana, 1% (n = 1) as Andhra Pradesh, and 1% (n = 1) as Kerala. The survey failed to generate responses from Puducherry. Seventy-seven percent (n = 102) of respondents work in private hospitals, 18% (n = 24) in government hospitals, 3% (n = 4) in non-government organizations, and 2% (n = 2) in clinics. Thirty-three (n = 44) percent identified their unit of work as ICU, 22% (n = 29) as emergency room, 27% (n = 35) as in-patient wards, and 27% (n = 36) as outpatient units. Thirty-two respondents reported working in more than 1 unit. Thirty-three respondents chose the other option and identified “operation theatre,” “clinical laboratory,” “community,” “palliative care,” “urology,” “radiology,” “labor room,” “nephrology,” and “obstetrics and gynecology” as their units.
Respondents belonged to various specialties of medicine. Notably, 28% (n = 37) identified their specialty as general medicine, 13% (n = 17) as anesthesiology, 9% (n = 12) as intensive care, 6% (n = 8) as cardiology, 6% (n = 8) as obstetrics and gynecology, 6% (n = 8) as pediatrics, and 6% (n = 8) as general surgery. Respondents belong to other specialties (neurology, orthopedics, and nephrology) were five or less.
Of 132 respondents, 61% (n = 81) stated that they were either treating or anticipate treating patients with COVID-19 and 39% (n = 51) reported that they did not foresee treating patients with COVID-19. A significant association was found between “compliance with the ICMR recommendation” and “treating or anticipate treating patients with COVID-19” (χ2 P = 0.003). Nearly 36% (n = 29) of respondents who treat/ anticipate treating COVID patients took HCQ prophylaxis, while only 14% (n = 7) complied with the recommendation in the other group (do not anticipate treating patients with COVID-19).
As 39% of the respondents reported that they did not treat or anticipate treating patients with COVID, understandably, their views may not provide an accurate reflection of the perception of health care workers view on ICMRs prophylaxis recommendation. Hence, their responses were removed from further analysis. Further, one respondent who selected the other option identified that he/she did not follow the recommendation because he/she has diabetes-related retinopathy. Hence, his/her response was also removed. Of the remaining 80 respondents, Figure 1 shows the number of respondents who took the HCQ prophylaxis. Figure 2 identifies the reasons for not taking the HCQ prophylaxis as reported by the respondents. Respondents were permitted to select more than 1 response and identify additional reasons through the “other” option for this item. Twenty respondents chose the “other” option. Their responses indicated concerns about side effects and lack of evidence.
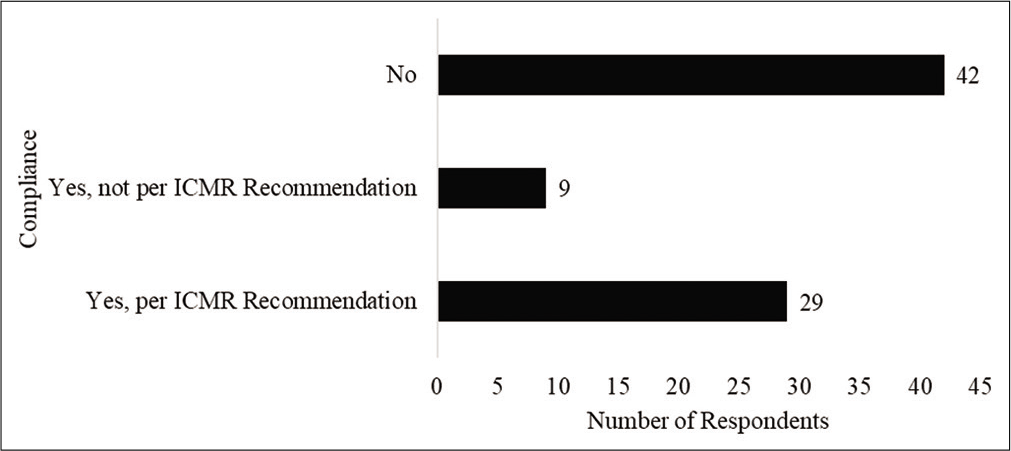
- Compliance of respondents with chemoprophylaxis.
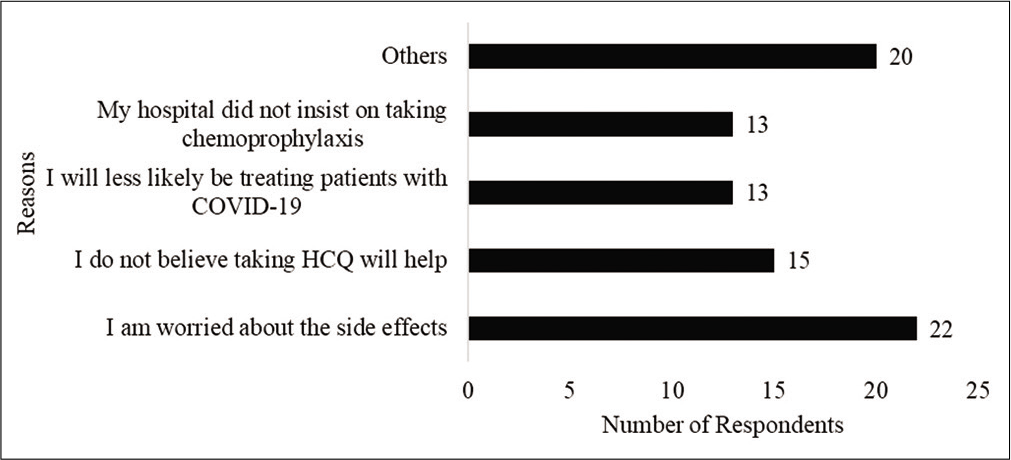
- Reasons for non-compliance with chemoprophylaxis.
Figure 3 presents respondents level of agreement to the statement of “taking HCQS prophylaxis will help a health-care professional feel safe when providing care to patients with COVID-19.” Interestingly, despite the agreement, only 15 of the 32 respondents who expressed agreement reported compliance with the prophylaxis. Two respondents who expressed disagreement and one respondent who expressed strong disagreement reported compliance with the recommendation.
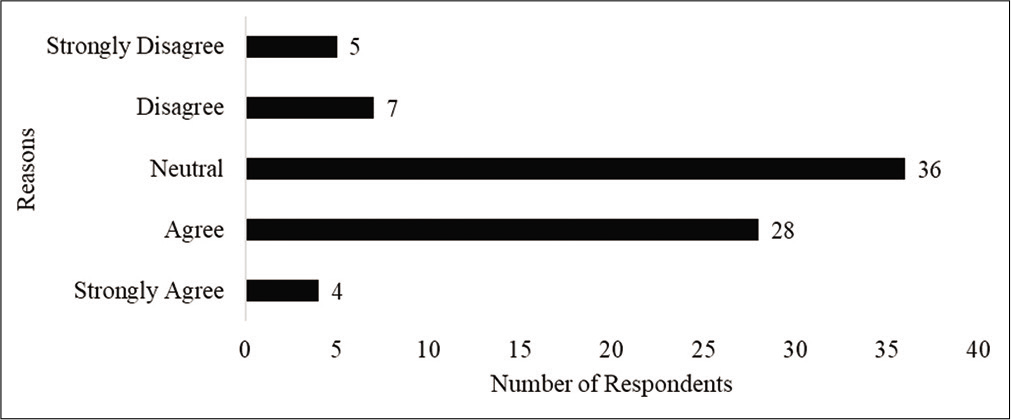
- Respondents level of agreement to the statement of HCQ being safe and effective.
Fifty-six respondents responded to the final open-ended item. The following themes emerged from the analysis of their responses: “Lack of evidence to support the efficacy of HCQS,” “side effects,” “lack of clarity,” and “must be taken if treating patients with COVID-19.” These themes were elaborated in the subsequent section.
DISCUSSION
The results of this study suggest that nearly half of frontline health care workers in South India, regardless of gender, geographical region (state), practice setting, and specialty did not comply with the HCQ chemoprophylaxis as recommended by the ICMR. Forty-seven percent (n = 38) of respondents reported taking the HCQ, of which, nine respondents did not follow the dosage recommendation made by the ICMR. This finding aligns with what has been reported in the literature. In a Delhi-based study, the authors found that doctors showed least acceptance of HCQ chemoprophylaxis.[17]
The test of association performed between “compliance with the recommendation” and “gender” (χ2 P = 0.68), “geographical region” (χ2 P = 0.21), “practice setting” (χ2 P = 0.24), and “specialty” (χ2 P = 0.78) yielded non-significant probability values.
Forty-three percent (n = 15) of respondents who work in the ICU stated that they took the chemoprophylaxis as per ICMRs recommendation. However, this percentage declined to 37 (n = 7) in outpatient clinics, 30 (n = 6) among respondents who work in emergency rooms, and 28 (n = 5) in inpatient wards. This suggests that practitioners were more compliant with the recommendation when the exposure risk is high.
Reasons for non-compliance
Concerns about side effects
Twenty-two respondents cited side effects as the reason for non-compliance. Their comments revealed that they were more concerned about cardiac side effects. For instance, several respondents commented “please do electrocardiogram (ECG) before the second dose,” “it prolongs QT interval,” “serial monitoring of ECGs,” “people with QT prolongation should not take the medicine,” and “consult a cardiologist before taking the right dosage of HCQ.” The concerns of respondents are not baseless as the HCQ has been reported to have gastrointestinal, cardiac, hepatic, psychological, sensory, and metabolic side effects.[18] Nagaraja et al. found a higher incidence of adverse events, mostly gastrointestinal, among health care workers who took the HCQ prophylaxis.[19] Bhattacharya et al. (2020) reported that one in five health care workers who complied with the HCQ chemoprophylaxis developed mild side effects.[17] Dhamija et al. reported that 36% of the respondents in their global survey reported side effects due to HCQ/chloroquine prophylaxis.[20] On the contrary, one study found no QT prolongation in health care workers who complied with the ICMRs chemoprophylaxis recommendation.[21]
Lack of evidence
Several participants stated that they did not take the chemoprophylaxis as there is no conclusive evidence on the efficacy of HCQ in preventing the infection. Some of their comments were as follows: “Efficacy not proven,” “without evidence-based medicine it’s not worth taking a tablet with so many life-threatening side effects,” “preliminary reports of efficacy are from press releases or small studies,” “there is no solid scientific evidence,” and “all studies demonstrating the supposed efficacy of HCQS are low power, p-hacked studies.” As discussed before, the literature on HCQs efficacy in equivocal.[3-6]
Lack of clarity
Some respondents’ comments (“want to know more about contraindications and drug interactions” and “concerns are more about its interaction with other drugs”) reveal that they were unclear about the recommendation and in need of more information on drug interactions and the rationale behind the recommendation.
Not treating patients with COVID
Several respondents believed that the HCQ prophylaxis may be helpful for health care workers who treat patients with COVID-19 than those do not treat. This was evident in their comments such as “good for people who are working as primary contact with infected patients,” “I think it will help for people working against this COVID-19,” and “can be given for young doctors with no co morbidity when working in frontline.” In addition, as shown in Figure 2, 13 respondents identified that they did not take the chemoprophylaxis because they will less likely be treating patients with COVID-19.
Strategies to promote compliance with prophylaxis recommendations
Provide clarity
The health authorities may provide clarity by publishing detailed guidelines on potential side effects when making prophylaxis recommendations. They should strive to provide clear instructions in multiple languages, conduct awareness activities, discuss the effectiveness, explain the prophylaxis schedule, and issue guidelines on cessation.[17] Further, they shall collect and publish ongoing data in terms of efficacy of the recommendations. Recommendations without clarity may lead to adverse outcomes as strong statements televised through media may influence the health care workers perception and instill a false sense of confidence. State medical councils and associations must be looped in to communicate the guidelines and educate organizations such as hospitals.
Consult stakeholders when making recommendations
All relevant parties such as representatives from different professional groups and organizations, and hospital administrators may be invited for a discussion when finalizing recommendations. This may encourage the buy-in of stakeholders and in-turn increase the compliance with the recommendation. During this consultation, it is essential that the authority making this recommendation provides adequate rationale behind the decision and answers all stakeholder inquiries. Further, this consultation opportunity could also be used for dissemination of information, planning for successful implementation of recommendations, and identify strategies to avoid misuse/abuse of recommendations.
Support recommendations with evidence
Contemporary health-care practitioners prefer information backed by evidence. Hence, it is critical that health authorities critically appraise all available evidence when finalizing the recommendations. Further, they may make available the appraised evidence for interested stakeholders on a web platform. If the evidence is equivocal or inconclusive, it may be wise to defer the recommendation while seeking for more conclusive evidence. The health authorities must realize that it will only create confusion among stakeholders if a recommendation made by one organization based on the positive findings of equivocal evidence is contradicted by another organization based on the negative findings of equivocal evidence.
Limitations
The major limitation of this study is the sampling methods and low number of responses. Given that the authors shared the link with the contacts in their network, the survey link may not have reached the wider audience. Since the survey did not ask respondents to identify the title, there are no data on how many of the respondents were physicians, nurses, or physician assistants. However, the authors believe that around 75% of responses were from physicians and 20% from nurses based on the list of people with whom they shared the survey link. A mail or telephone survey may have increased the number of responses. An in-depth survey with more questions (what their professional title is, what could have increased their compliance and confidence with ICMRs recommendation, etc.) may have yielded additional insights.
Recommendations
Synthesizing available evidence and conducting a focus group inviting stakeholders from different professional groups may yield valuable insights related to the HCQ chemoprophylaxis.
CONCLUSION
Slightly more than half of frontline health-care professionals in South India did not adhere to the ICMRs recommendation on the HCQ chemoprophylaxis due to concerns about side effects, lack of evidence on the efficacy of HCQ in preventing COVID-19, and lack of clarity. Evidence-based recommendations, publication of detailed guidelines by state and central health authorities, and stakeholder consultation may increase the compliance with prophylaxis recommendations.
Acknowledgment
The authors express their sincere gratitude to all respondents who spared their time to respond to the survey.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University [Internet] 2020. Baltimore: JHU; Available from: https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 [Last accessed on 2020 Dec 24]
- [Google Scholar]
- Advisory on the Use of Hydroxychloroquine as Prophylaxis for SARS-CoV-2 Infection New Delhi: ICMR; 2020.
- [Google Scholar]
- A randomized trial of hydroxychloroquine as postexposure prophylaxis for COVID-19. N Engl J Med. 2020;383:517-25.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxychloroquine for the prevention of COVID-19-searching for evidence. N Engl J Med. 2020;383:585-6.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of hydroxychloroquine vs placebo for pre-exposure SARS-CoV-2 prophylaxis among health care workers: A randomized clinical trial. JAMA Intern Med. 2020;181:195-202.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxychloroquine as pre-exposure prophylaxis for Coronavirus disease 2019 (COVID-19) in healthcare workers. A randomized trial. 2020. Clin Inf Dis. :ciaa1571. Available from: https://www.medrxiv.org/content/medrxiv/early/2020/09/21/2020.09.18.20197327.full.pdf [Last accessed on 2020 Dec 24]
- [Google Scholar]
- Low-dose hydroxychloroquine therapy and mortality in hospitalised patients with COVID-19: A nationwide observational study of 8075 participants. Int J Antimicrob Agents. 2020;56:106144.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for mortality in patients with COVID-19 in New York city. J Gen Intern Med. 2020;36:17-26.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396-403.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of 3, 737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis. Travel Med Infect Dis. 2020;36:101791.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020;383:2030-40.
- [CrossRef] [PubMed] [Google Scholar]
- WHO Discontinues Hydroxychloroquine and Lopinavir/Ritonavir Treatment ARMS for COVID-19. 2020. Geneva: World Health Organization; Available from: https://www.who.int/news/item/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19 [Last accessed on 2020 Dec 24]
- [Google Scholar]
- Hydroxychloroquine with or without azithromycin in mild-to-moderate COVID-19. N Engl J Med. 2020;383:2041-52.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxychloroquine. 2020. Available from: https://www.youtube.com/watch?v=2uzxhnuviro [Last accessed on 2020 Dec 24]
- [Google Scholar]
- Online Survey Development Software. 2020. San Mateo. CA: SurveyMonkey; Available from: https://www.surveymonkey.com [Last accessed on 2020 Jun 30]
- [Google Scholar]
- Internet, Mail, and Mixed Mode Surveys: The Tailored Design Method (3rd ed). New Jersey: John Wiley & Sons; 2009.
- [Google Scholar]
- Chemoprophylaxis of COVID-19 with Hydroxychloroquine: A Study of Health Care Workers Attitude. 2020. Available from: https://www.medrxiv.org/content/medrxiv/early/2020/06/12/2020.06.11.20126359.full.pdf [Last accessed on 2020 Dec 25]
- [Google Scholar]
- WebMD Hydroxychloroquine Sulfate. 2020. Available from: https://www.webmd.com/drugs/2/drug-5482/hydroxychloroquine-oral/details [Last accessed on 2020 Dec 25]
- [Google Scholar]
- HyPE study: Hydroxychloroquine prophylaxis-related adverse events' analysis among healthcare workers during COVID-19 pandemic: A rising public health concern. J Public Health (Oxf). 2020;42:493-503.
- [CrossRef] [PubMed] [Google Scholar]
- Awareness and Impact of Hydroxychloroquine/Chloroquine Prophylaxis among the Healthcare Workers During the COVID-19 Pandemic: An Observational Study. 2020
- [CrossRef] [Google Scholar]
- HCQ prophylaxis in COVID-19 did not show any QTc prolongation in healthcare workers. Indian Heart J 2020
- [CrossRef] [PubMed] [Google Scholar]






