Translate this page into:
Live-attenuated oral polio vaccine as a potential source of protection against COVID-19 – Review of literature

*Corresponding author: Vishal Rao US, Dean, Centre for Academic Research, HCG Cancer Hospital, Bengaluru - 560 001, Karnataka, India. drvishalrao@hcgel.com
-
Received: ,
Accepted: ,
How to cite this article: Rao V, Rao U, Kunigal SS, Kannan S, Kumar J, Gulia A. Live-attenuated oral polio vaccine as a potential source of protection against COVID-19 – Review of literature. Indian J Med Sci 2021;73(1):41-7.
Abstract
The widespread surge in COVID-19 infections has caused an overwhelming rise in the number of hospital admissions and patient deaths. Massive research efforts are underway globally to develop COVID-19 vaccines. For the newly developed vaccines, given that safety beyond the trial population and the worldwide accessibility remains to be determined, there is also an opportunity to explore repurposing the pre-existing safe vaccines like the oral polio vaccine (OPV) leveraging their potential to provide cross-protection. The plausible mechanisms by which OPV might provide partial cross-immunity against SARS-CoV-2 include inhibition of PVR-TIGITCD226 axis and stimulation of trained innate immunity. Inhibition of PVR-TIGIT-CD226 axis by OPV unleashes the immunosuppressive effects of TIGIT, thus priming the immune system against the invading pathogen. Stimulation of trained innate immunity by OPV due to metabolic reprogramming and epigenetic modifications provides partial protection. This paper reviews the literature about live-attenuated OPV as a potential source of protection against COVID-19 and highlights the need for randomized, multicentric trials in India.
Keywords
COVID-19
Oral polio vaccine
Immunity
SARS-CoV-2
INTRODUCTION
COVID-19 is caused by the novel coronavirus SARS-CoV-2, which originated in Wuhan, China, in December 2019.[1] The WHO has declared it a pandemic on March 11, 2020, as it has spread to more than 114 countries infecting millions of individuals throughout the world.[2] It has infected 51,480,441 people globally with 1,272,426 death worldwide as on November 11, 2020.[3] COVID-19 has varied clinical presentation ranging from mild asymptomatic infections to acute respiratory distress syndrome with multiple organ involvement.[1] COVID-19 has posed a serious health-care emergency throughout the world, even more so for a developing country like India with limited health-care resources and non-uniform health-care infrastructure. As we are in the midst of the pandemic, there is an urgent need to explore the possibility of repurposing already available vaccines. Recent epidemiological data suggest that the use of live-attenuated vaccines such as Bacillus Calmette-Guérin (BCG), measles vaccine, and oral polio vaccine (OPV) may result in the induction of non-specific effects on the immune system following one or two doses of these vaccines and may protect against other types of viruses.[4-10] Moreover, retrospective analysis done at Mayo clinic has shown that individuals with a history of previous immunization with OPV across 1-, 2-, to 5-year span have lower rate of SARS-CoV-2 infection compared to non-vaccinated individuals.[11]
In a retrospective analysis[12] of large cohort of Danish children, OPV was associated with lower rate of overall hospital admission with other non-polio-related infections and significantly lower rates of lower respiratory infection. There is substantial clinical evidence endorsing the potential health benefits of live-attenuated vaccines (LAVs) like OPV; it provides protection against heterologous infections with unrelated pathogens and reduces overall mortality.[12-15] The possibility of repurposing the existing LAV such as OPV and BCG against COVID-19 is being actively explored as LAV will offer partial immunity till the safety profile of the new vaccine against SAR-CoV-2 has been established in the population.[16-19]
A systematic review[9] conducted by a research team commissioned by the World Health Organization analyzed 68 papers on the topic, many of which came from studies conducted under the Bandim Health Project in Guinea-Bissau. The study concluded that BCG and Measles vaccines reduce overall mortality by more than would be expected through their effects on the diseases they prevent. Some of the research the team evaluated linked the measles vaccine with 50% lower risk of death from any cause.[9] Although the molecular mechanism is not established, it might involve indirect immunomodulation mediated through measles vaccines.[20] Since July 15, 2020, researchers from the Bandim Health Project in Guinea-Bissau are conducting a Phase 4 cluster randomized trial on OPV as potential protection against COVID-19. The investigators are assessing the effect of providing OPV versus no vaccine to 3400 persons above 50 years of age. The trial will have the power to test the hypothesis that OPV reduces the combined risk of morbidity, admission, or death (composite outcome) by at least 28% over the subsequent 6 months.[21]
The most plausible reason for cross-immunity could be shared B-cell and T-cell epitopes among the two viruses, however, no similarity or overlap of B-cells and T-cells epitopes was found between SARS-CoV-2 and poliovirus (PV) in the study done by Reche.[22] The absence of molecular mimicry or similarity at the level of amino acid sequences favors the contribution of innate immunity in comparison to adaptive immunity toward partial protection provided by OPV against SARS-CoV-2.
This paper reviews the literature with regard to various immunomodulatory mechanisms mediated by OPV that could provide cross-protection against SARS-CoV-2 infection. Further, the paper also recommends that randomized, multicentric trials in India be conducted at the earliest.
HYPOTHESIZING PLAUSIBLE MECHANISMS TO EXPLAIN NON-SPECIFIC IMMUNOMODULATION BY OPV THROUGH LITERATURE SYNTHESIS
Innate immune response is elicited after identification of pathogen-associated molecular pattern (PAMP) of the invading microbe by pattern recognition receptors (PRRs) which leads to the activation of nuclear factor-kappa B cells (NF-kB) pathway and subsequent release of cytokines and interferons that kill the invading pathogen.[23,24]
Conventionally, innate immune responses were believed to be of short duration, but the recent concept of “Trained Innate immunity” proposes that they persist for months to years after initial stimulus, despite the short lifespan of only days in circulation of macrophages and natural killer cells (NK cells).[25] This could be attributed to myelopoiesis bias that favors progenitor cells with transcriptional, epigenetic, and functional changes of heightened immune response to be released in the peripheral circulation.[17,26] LAVs have higher immunogenicity in comparison to inactivated vaccine and they stimulate the innate immune system through PRRs in more efficient manner than inactivated vaccine where innate immunity stimulation is limited in the absence of microbial replication.[27,28] In case of BCG vaccination, the enhanced trained innate immune response was observed until 1 year, although the effect was less pronounced after 3 months.[25] Monocytes extracted after 1 year showed increased expression of PRR like TLR4.[25]
The epithelial-immune cell interactions have been reported to play an important role in determining the severity of COVID-19 infections.[29] Since, poliovirus receptor (PVR) has already been implicated in modulating immune response, we sought to explore the cell/tissue types expressing both these receptors at single-cell resolution.
The coexpression of ACE2 and PVR was observed in the respiratory tract, small intestine, kidney, heart, and pancreas at the protein and single-cell level.[30]
We correspondingly also observe the coexpression of PVR and ACE2 in the immature enterocytes[31,32] of the intestine which might be relevant in the context of SARSCoV-2 infection as many gastrointestinal complications are observed in COVID-19 patients.[1] Interestingly, coexpression of ACE2 and PVR was also observed in the cardiomyocytes[33] in the heart tissue. Acute pancreatitis has been reported in a few cases of COVID-19.[34] However, few clinical trials are ongoing to study the effect of COVID-19 on other organs and also its long-term sequelae.[35,36] The expression of ACE2 coupled with the presence of PVR, which is known to play an important role in regulating immune response, might provide mechanistic insights into OPV-mediated protection against SARS-CoV-2 as respiratory, gastrointestinal, and cardiac symptoms are commonly seen in patients of COVID-19.[1]
On the basis of an exhaustive literature review, we propose the following hypotheses which might explain the non-specific immunomodulation by which OPV might confer partial immunity against SARS-CoV-2.
INHIBITION OF PVR-TIGIT AXIS BY OPV LIFTS OFF THE IMMUNE INHIBITORY EFFECT ON NK CELLS, T-CELLS, AND MACROPHAGES
CD155 was earlier known as PVR due to its binding to human PV. PVR is a cell adhesion molecule of the nectin/nectin-like family with a wide range of functions including cell adhesion, migration, and immune response modulation.[37-40] It is also the main binding receptor for the poliovirus which facilitates its entry inside the cell.[41] Association of TIGIT with PVR inhibits immune cells including NK cells, T-cells, and macrophages.[42,43] TIGIT (T-cell immunoglobulin with immunoreceptor tyrosine-based inhibitory motif domain) is a recently discovered immune checkpoint molecule belonging to immunoglobulin superfamily with potential antiviral and anti-tumor activity due to its immune inhibitory effect on T cells and NK cells.[42,44] It interacts with the costimulatory receptor DNAM-1 and the inhibitory receptors TIGIT and CD96, resulting in either immune cell activation or inhibition, respectively.[38,42] Binding affinity of PVR/CD155 is higher for TIGIT (inhibitory molecule) compared to CD226 (costimulatory molecule).[38,42] Hence, CD155/PVR modulates the immune response depending on binding affinity and downstream receptor binding at the cell surface.[38]
OPV (LAV) inhibits PVR and its downstream binding immune inhibitory signals through binding with TIGIT, thus keeping the T cells and NK cells in active state by lifting off inhibitory signals to acts against the invading pathogen.[43]
Hence, it is logical to propose that OPV administration may be beneficial against COVID-19, by causing inhibition of PVR and its associated immunosuppressive network [Figure 1.1].
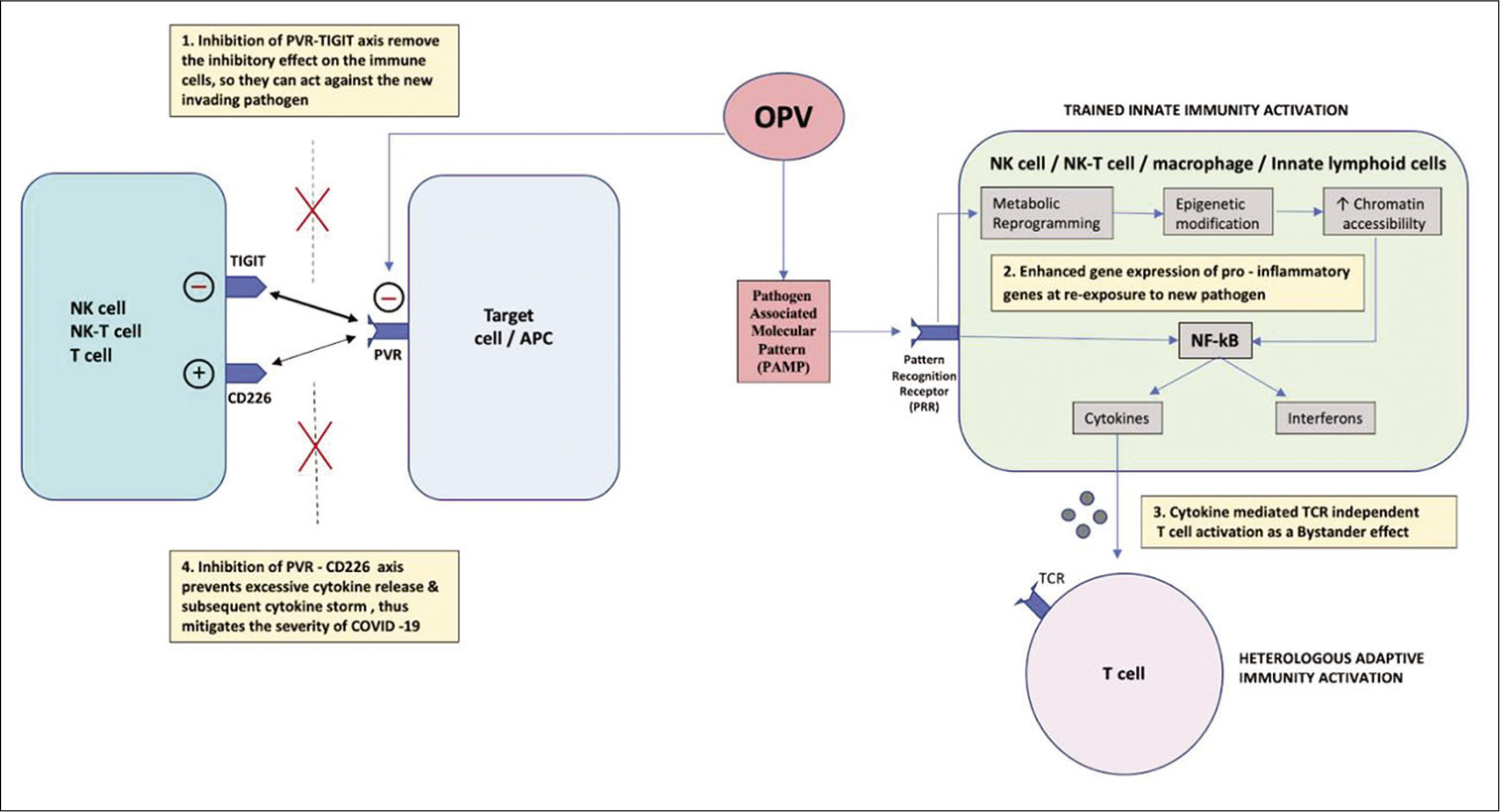
- Mechanisms by which OPV confers immunity against SARS-CoV-2 and mitigates its severity (1) OPV inhibits PVR/CD155 receptor and its downstream binding with TIGIT, thereby removing its immuno-inhibitory effect on NK cells and T cells, so they are in active state to combat invading pathogen. (2) OPV stimulates trained innate immunity by activating PRR through PAMP. PRR initiates metabolic reprogramming and epigenetic modifications which increase the chromatin accessibility for rapid binding of transcription factor at promoter and enhancer region of pro-inflammatory gene, thus resulting in enhanced gene expression and heightened response on re-exposure to invading pathogen by increase release of cytokines and interferon through activation of NF-kB pathway. (3) Increase cytokine release in the adjacent microenvironment mediates TCR-independent activation of T-cell as a bystander effect, despite the weak binding of PV antigen to TCR. (4) OPV inhibits PVR and its binding to CD226 which is a costimulatory molecule, thereby preventing excessive cytokine release and subsequent cytokine storm, thus mitigating the severity of COVID-19. OPV: Oral poliovirus, APC: Antigen-presenting cell, TCR: T-cell receptor, NK cell: Natural killer cell, NK-T cell: Natural killer T-cell, NF-kB: Nuclear factor kappa beta, PV: Poliovirus, PVR: Poliovirus receptor, TCR: T-cell receptor, PAMP: Pathogen-associated molecular pattern, PRR: Pattern recognition receptor.
OPV AND OTHER LAVS UPREGULATE THE TRAINED INNATE IMMUNITY BY EPIGENETIC MODIFICATIONS AND METABOLIC PROGRAMMING
Trained innate immunity is a relatively new concept of enhanced response and immunological memory of innate immune cells[45,46] (NK cells, NK-T cells, macrophages, and innate lymphoid cells) facilitated by metabolic reprogramming and epigenetic modifications on second exposure to pathogen.[47,48] Metabolic reprogramming manages the accumulation or depletion of certain metabolites in the cells that function as cofactors for enzymes involved in epigenetic modification,[5,49,50] such as histone methylation and acetylation, which results in enhanced chromatin accessibility for rapid binding of transcription factor at enhancer and promoter regions and easier transcription of genes critical for antimicrobial responses.[49] Therefore, trained innate immunity enables faster and increased responsiveness that could persist for months to year[25] in cases of subsequent infection with unrelated pathogen due to persistence of epigenetic modifications.[16] Therefore, we propose that these “Trained innate immune cells,” generated on initial administration of live-attenuated OPV, provide protection against SARS-CoV-2 [Figure 1.2].
OPV MAY CONTRIBUTE TO TCR-INDEPENDENT T-CELL ACTIVATION MEDIATED THROUGH CYTOKINES AS A BYSTANDER EFFECT
Heterologous immunity is the immunity developed against a pathogen after exposure or infection with an unrelated pathogen.[51] It develops mainly due to cross-reactivity of T-cell or antibodies due to molecular mimicry.[49] The other reason for T-cell activation against unrelated pathogen is bystander activation where signals from ongoing immune response following exposure to different pathogen may raise the cytokine levels which, in turn, leads to TCR-independent T-cell activation.[47,48,51,52] The signals mainly include cytokines like interleukin-2, which facilitate T-cell activation and increase responses of unrelated plasma cells.[52,53] A recent study has implicated cytokines such as type-1 interferon, IL-15, and IL-18 to be playing a major role in TCR-independent T-cell activation as a result of bystander effect.[54]
As no similarity was found between SARS-CoV-2 and PV after exhaustive search for n-mers, peptides, and T and B cells epitopes, we have excluded the cross-immunity on account of molecular mimicry as a potential reason of heterologous adaptive immune response generated by OPV against SARS-CoV-2. Hence, we postulate that heterologous adaptive T-cell immune response against SAR-CoV-2 post-OPV administration could be attributed to bystander effect on T-cell activation due to cytokines such as IL2, IL15, IL18, and IFN gamma [Figure 1.3].
OPV MIGHT REGULATE PVR-CD226 MEDIATED CYTOKINE RESPONSE
It has been observed and previously reported that administration of LAVs like BCG can mitigate the severity of disease in case of COVID-19.[16,28] The results from the ongoing clinical trial would shed more light on the effect of BCG on the disease severity in COVID-19.[55] Similarly, OPV can also reduce the severity of COVID-19 in vaccinated individuals on exposure to SARS-CoV-2. One plausible cause is the reduced viremia and symptoms in case of COVID-19 patients who were previously vaccinated with LAV on account of trained innate immunity[28] and inhibition of PVR-TIGIT inhibitory effect on NK cells and macrophages. PVR also modulates the immune response depending on ligand concentration and binding affinity of downstream signaling molecules at the cell surface,[42,43] which might explain the cause of varied immune response observed in COVID-19 patients ranging from immunosuppression to hyperinflammation and cytokine storms.[56]
The other important mechanism for mitigating the COVID-19 severity in individuals with previous OPV administration could be inhibition of PVR-CD226 binding. CD226/DNAM1 is a costimulatory molecule expressed on NK cells, T-cells, and dendritic cells which on binding with PVR activate NK and T-cells to release cytokine and may lead to cytokine storm on persistent stimulation.[43] Recently, CD226 G allele was linked to critical COVID-19 illness based on documented evidence of severe influenza symptoms in patients with CD226G allele which has higher binding affinity to PVR/CD155 receptor.[57] As OPV inhibits PVR and its downstream binding with CD226, it can prevent excessive release of cytokine and subsequent cytokine storm in COVID-19 patients. Thus, we hypothesize that OPV administration might mitigate COVID-19 disease severity as it can reduce the viremia and also the prevent cytokine storm by binding to PVR and inhibiting its downstream binding with CD226 which stimulate immune cells to release cytokines [Figure 1.4].
Alternatively, it has also been reported previously that the 3A protein of PV suppresses the expression of IL-6 in the MG-63 cell line model.[58] Since IL-6 has already been reported to be a marker for severe COVID-19,[59] lower IL-6 production on poliovirus infection could also potentially prove beneficial.
Although all LAVs confer some immunity against the new pathogen, bivalent OPV (bOPV) may be better suited compared to other LAV due to inhibition of PVR binding with TIGIT and CD226, which may have beneficial effect in potentiating innate immune response to fight against SARCoV-2 and also mitigate disease severity.[43] OPV has in place pre-existing robust infrastructure for vaccine supply, storage and surveillance, and also an easy oral route of administration which makes it more preferable compared to other LAV such as BCG and measles vaccine.
bOPV is LAV containing PV strain 1 and 3, PV strain 2 has been removed from the bOPV in 2016 by Global Polio Eradication Initiative in the light of vaccine-associated paralytic poliomyelitis (VAPP) and circulating vaccine-derived polioviruses cases associated with PV2 strain.[60-63] IPV stimulates predominantly humoral adaptive immune response whereas bOPV stimulates innate immune response and intestinal immunity in addition to adaptive humoral immunity as it is a LAV.[61] Unlike IPV, bOPV also provides intestinal mucosal immunity which is responsible for heterologous lymphocyte activation as gut epithelium has a reservoir of innate lymphoid cells which mimic the function of T-helper 2 cells.[64]
Therefore, bOPV is more suitable for vaccine repurposing compared to IPV as it stimulates the innate immune system and causes heterologous lymphocyte activation to provide partial immunity against SAR-COV-2. bOPV is safe to administer as it does not contain the PV2 strain responsible for most cases of VAPP.[62,63]
Hence, there is an urgent need to start clinical trials to look into potential benefits of repurposing OPV against SARCoV-2 until a more effective vaccine is available. OPV shedding in stool samples was detected for more than 3 weeks in immunological naive children,[65] however, prior vaccination with OPV/IPV reduces both the number of samples showing viral shedding and also its duration in the stool samples.[65] The clinical trial will also be helpful in monitoring the viral replication and its shedding in stool samples to decide on the timing and dosage of vaccination for providing immunity against SARS-CoV-2.
With the fulminant resurgence of COVID-19 in India, there has been an increase in the number of pediatric COVID-19 hospitalizations in certain parts of the country.[66] It can be argued that most of these cases have received OPV and BCG vaccination as per the National Immunization Schedule, and thus, the hypothesized cross-protection from OPV against SARS-CoV-2 may indeed not occur. However, from anecdotal evidence, the relative severity of COVID-19 is lesser in pediatric cases compared to adults even in the second wave, and it could very well be that the innate immune system activation has a role to play in the partial immunity against severe COVID-19 in pediatric cases. Further, there is virtually no evidence on the cross-protection conferred by OPV in adults, which is the age group of primary interest.
India is currently facing major shortages to execute its ambitious universal COVID-19 vaccination drive after having entered its third phase that includes all adults above 18 years of age. In such circumstances, reactivation of the innate immune system with a LAV like OPV could theoretically provide partial immunity against SARS-CoV-2 until COVID-19 vaccines are widely available, and could also augment the subsequent immune response through a combination of the OPV, a LAV, and an adenovirus vector-based or inactivated vaccine categories of the three COVID-19 vaccine candidates that currently are approved for administration in India.
POLICY RECOMMENDATIONS FOR GOVERNMENT OF INDIA
Based on our extensive review of literature and the theories we proposed, the following recommendations are made;
Conduct an epidemiological study to draw inferences on the correlation between polio immunization coverage and overall morbidity and mortality for COVID-19 in India
Conduct a cluster randomized trial on the effect of OPV on adults to test whether OPV reduces the risk of morbidity, hospital admission, or mortality
Partner with the Bandim Health Project to conduct a multicentric trial on LAVs and the benefits they confer on rural and low socioeconomic populations.
CONCLUSION
The COVID-19 pandemic has already overburdened our health-care system. Escalating cases and scarcity of hospital beds and other resources have further worsened the situation, thereby bringing health-care facilities on the brink of collapse and catastrophe. In view of the supportive clinical literature and strong molecular evidence of various plausible mechanisms by which OPV confers immunity including inhibition of PVR-TIGIT-CD226 axis, stimulation of trained immunity, and cytokine-induced TCR-independent T-cell activation, it is imperative to start clinical trials at the earliest to study the benefits of OPV in providing immunity against SARS-CoV-2, as to also monitor its effects on the severity of disease in COVID-19 patients.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Vishal Rao is on the National Advisory Board and Dr. Ashish Gulia is the Editor of this journal.
References
- Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020
- [CrossRef] [Google Scholar]
- WHO Characterizes COVID-19 as a Pandemic. 2020. Geneva: World Health Organization; Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen [Last accessed on 2020 Oct 03]
- [Google Scholar]
- Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. J Leukoc Biol. 2015;98:347-56.
- [CrossRef] [PubMed] [Google Scholar]
- Non-specific effects of vaccines: Current evidence and potential implications. Semin Immunol. 2018;39:35-43.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic Review of the Non-specific Effects of BCG, DTP and Measles Containing Vaccines Geneva: World Health Organization; 2014.
- [Google Scholar]
- Unravelling the nature of non-specific effects of vaccines a challenge for innate immunologists. Semin Immunol. 2016;28:377-83.
- [CrossRef] [PubMed] [Google Scholar]
- Beneficial nonspecific effects of oral polio vaccine (OPV): Implications for the cessation of OPV? Clin Infect Dis. 2017;65:420-1.
- [CrossRef] [PubMed] [Google Scholar]
- Association of BCG, DTP, and measles containing vaccines with childhood mortality: Systematic review. BMJ. 2016;355:i5170.
- [CrossRef] [PubMed] [Google Scholar]
- Nonspecific effects of oral polio vaccine on diarrheal burden and etiology among Bangladeshi infants. Clin Infect Dis. 2017;65:414-9.
- [CrossRef] [PubMed] [Google Scholar]
- Exploratory analysis of immunization records highlights decreased SARS-CoV-2 rates in individuals with recent non-COVID-19 vaccinations. medRxiv. 2021;11:4741.
- [CrossRef] [PubMed] [Google Scholar]
- Oral polio vaccination and hospital admissions with non-polio infections in Denmark: Nationwide retrospective cohort study. Open Forum Infect Dis. 2016;3:ofv204.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of oral polio vaccine at birth on infant mortality: A randomized trial. Clin Infect Dis. 2015;61:1504-11.
- [CrossRef] [PubMed] [Google Scholar]
- National immunization campaigns with oral polio vaccine reduce all-cause mortality: A natural experiment within seven randomized trials. Front Public Health. 2018;6:13.
- [CrossRef] [PubMed] [Google Scholar]
- Oral polio vaccination and low case fatality at the paediatric ward in Bissau, Guinea-Bissau. Vaccine. 2004;22:3014-7.
- [CrossRef] [PubMed] [Google Scholar]
- BCG-induced trained immunity: Can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-7.
- [CrossRef] [PubMed] [Google Scholar]
- Trained immunity: A tool for reducing susceptibility to and the severity of SARS-CoV-2 infection. Cell. 2020;181:969-77.
- [CrossRef] [PubMed] [Google Scholar]
- Considering BCG vaccination to reduce the impact of COVID-19. Lancet. 2020;395:1545-6.
- [CrossRef] [Google Scholar]
- Can existing live vaccines prevent COVID-19? Science. 2020;368:1187-8.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science. 2015;348:694-9.
- [CrossRef] [PubMed] [Google Scholar]
- OPV as Potential Protection against COVID-19. 2000. Identifier NCT04445428. Available from: https://www.clinicaltrials.gov/ct2/show/record/NCT04445428 [Last accessed on 2020 Aug 29]
- [Google Scholar]
- Potential cross-reactive immunity to SARS-CoV-2 from common human pathogens and vaccines. Front Immunol. 2020;11:586984.
- [CrossRef] [PubMed] [Google Scholar]
- Innate immune recognition: Mechanisms and pathways. Immunol Rev. 2000;173:89.
- [CrossRef] [PubMed] [Google Scholar]
- Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J Innate Immun. 2014;6:152-8.
- [CrossRef] [PubMed] [Google Scholar]
- Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell. 2018;172:147-61.e12.
- [CrossRef] [PubMed] [Google Scholar]
- Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203:413-24.
- [CrossRef] [PubMed] [Google Scholar]
- Vaccine immunology In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines (6th ed). Philadelphia, PA: Saunders Elsevier; 2013. p. :14-33.
- [Google Scholar]
- COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol. 2020;38:970-9.
- [CrossRef] [PubMed] [Google Scholar]
- Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419.
- [CrossRef] [PubMed] [Google Scholar]
- Single-cell transcriptome analysis reveals differential nutrient absorption functions in human intestine. J Exp Med. 2020;217:e20191130.
- [CrossRef] [PubMed] [Google Scholar]
- Intra and inter-cellular rewiring of the human colon during ulcerative colitis. Cell. 2019;178:714-30.e22.
- [CrossRef] [PubMed] [Google Scholar]
- Single-cell reconstruction of the adult human heart during heart failure and recovery reveals the cellular landscape underlying cardiac function. Nat Cell Biol. 2020;22:108-19.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence, risk factors, and outcomes of hospitalized patients with COVID-19 presenting as acute. Gastroenterology. 2020;159:2226-8.e2.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term Impact of Infection With Novel Coronavirus (COVID-19) (LIINC) 2020. Identifier NCT04362150. Available from: https://www.clinicaltrials.gov/ct2/show/record/NCT04362150?view=record [Last accessed on 2020 Nov 23]
- [Google Scholar]
- A Longitudinal Study of COVID-19 Sequelae and Immunity. 2020. Identifier NCT04411147. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04411147 [Last accessed on 2020 Nov 23]
- [Google Scholar]
- Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9:603-15.
- [CrossRef] [PubMed] [Google Scholar]
- Poliovirus receptor: More than a simple viral receptor. Virus Res. 2017;242:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- The role of NK cell recognition of nectin and nectin-like proteins in tumor immunosurveillance. Semin Cancer Biol. 2006;16:359-66.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor intrinsic and extrinsic immune functions of CD155. Semin Cancer Biol. 2020;65:189-96.
- [CrossRef] [PubMed] [Google Scholar]
- Cellular receptor for poliovirus: Molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855-65.
- [CrossRef] [Google Scholar]
- TIGIT blockade: A multipronged approach to target the HIV reservoir. Front Cell Infect Microbiol. 2020;10:175.
- [CrossRef] [PubMed] [Google Scholar]
- Intoxication with endogenous angiotensin II: A COVID-19 hypothesis. Front Immunol. 2020;11:1472.
- [CrossRef] [PubMed] [Google Scholar]
- The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26:923-37.
- [CrossRef] [PubMed] [Google Scholar]
- Specific memory within innate immune systems. Trends Immunol. 2005;26:186.
- [CrossRef] [PubMed] [Google Scholar]
- Trained immunity: A memory for innate host defense. Cell Host Microb. 2011;9:355.
- [CrossRef] [PubMed] [Google Scholar]
- Harnessing the beneficial heterologous effects of vaccination. Nat Rev Immunol. 2016;16:392-400.
- [CrossRef] [PubMed] [Google Scholar]
- Virus-induced T cell-mediated heterologous immunity and vaccine development. Front Immunol. 2020;11:513.
- [CrossRef] [PubMed] [Google Scholar]
- Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352:aaf1098.
- [CrossRef] [PubMed] [Google Scholar]
- Metaboloepigenetics: Interrelationships between energy metabolism and epigenetic control of gene expression. J Cell Physiol. 2012;227:3169.
- [CrossRef] [PubMed] [Google Scholar]
- No one is naive: The significance of heterologous T-cell immunity. Nat Rev Immunol. 2002;2:417-26.
- [CrossRef] [PubMed] [Google Scholar]
- Bystander activation of irrelevant CD4+ T cells following antigen-specific vaccination occurs in the presence and absence of adjuvant. PLoS One. 2017;12:e0177365.
- [CrossRef] [PubMed] [Google Scholar]
- Maintenance of serological mem ory by polyclonal activation of human memory B cells. Science. 2002;298:2199e202.
- [CrossRef] [PubMed] [Google Scholar]
- The activation of bystander CD8+ T cells and their roles in viral infection. Exp Mol Med. 2019;51:1-9.
- [CrossRef] [PubMed] [Google Scholar]
- BCG Against Covid-19 for Prevention and Amelioration of Severity Trial (BAC to the PAST) 2020. Identifier NCT04534803. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04534803 [Last accessed on 2020 Nov 23]
- [Google Scholar]
- Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020;19:102567.
- [CrossRef] [PubMed] [Google Scholar]
- Association of severe influenza virus infections with CD226 (DNAM-1) variants. J Infect Dis. 2019;220:1162-5.
- [CrossRef] [PubMed] [Google Scholar]
- Poliovirus 3A protein limits interleukin-6 (IL-6), IL-8, and beta interferon secretion during viral infection. J Virol. 2001;75:8158-65.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146:128-36.e4.
- [CrossRef] [PubMed] [Google Scholar]
- Semi-annual Status Report: January to June 2016 Geneva, Switzerland: World Health Organization; 2016.
- [Google Scholar]
- Weekly Epidemiological Record. . 2014;9:73-92. Available from: http://www.who.int/wer/2014/wer8909.pdf?ua=1 [Last accessed on 2020 Oct 03]
- [Google Scholar]
- Vaccine-associated paralytic poliomyelitis: A review of the epidemiology and estimation of the global burden. J Infect Dis. 2014;210(Suppl 1):S380-9.
- [CrossRef] [PubMed] [Google Scholar]
- Vaccine-derived polioviruses. J Infect Dis. 2014;210(Suppl 1):S283-93.
- [CrossRef] [PubMed] [Google Scholar]
- Role of innate lymphoid cells and dendritic cells in intradermal immunization of the enterovirus antigen. NPJ Vaccines. 2019;4:14.
- [CrossRef] [Google Scholar]
- Effect of different vaccination schedules on excretion of oral poliovirus vaccine strains. J Infect Dis. 2005;192:2092-8.
- [CrossRef] [PubMed] [Google Scholar]
- More Children Covid Positive in Second Wave. 2021. Hindustan Times. Available from: https://www.hindustantimes.com/cities/pune-news/more-children-covid-positive-in-second-wave-101618657160956.html [Last accessed on 2021 May 02]
- [Google Scholar]






